Abstract
Heterostructured nanomaterials are of interest for many applications due to their unique properties, which depend on the identity of each individual component and the interface between them. However, the difficulty of controlling the synthesis of heterostructures and the interfaces between components limits their applications. Here we develop a colloidal synthetic method to prepare heterostructured intermetallic nanomaterials (iNMs) and engineer the interfaces between the individual components, based on the galvanic replacement mechanism and precisely controlled addition of precursors. Up to four distinct intermetallic phase-segregated segments could be combined in one nanoparticle. Nanometre-precision phase-segregated control along one dimension of iNMs was demonstrated by taking advantage of the layered growth pathway at the intermetallic–metal interfaces, leading to a maximum number of interfaces between different intermetallic phases. By adjusting the number of interfaces in a particle, we demonstrated systematic control of the interface-to-bulk ratio in heterostructured iNMs. This method offers great potential for preparing complex heterostructured nanomaterials with controlled interfaces, enabling the exploration of their properties and applications.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text and the supplementary materials.
References
Steimle, B. C., Fenton, J. L. & Schaak, R. E. Rational construction of a scalable heterostructured nanorod megalibrary. Science 367, 418–424 (2020).
Fenton, J. L., Steimle, B. C. & Schaak, R. E. Tunable intraparticle frameworks for creating complex heterostructured nanoparticle libraries. Science 360, 513–517 (2018).
Chen, P.-C. et al. Polyelemental nanoparticle libraries. Science 352, 1565–1569 (2016).
Sun, X. et al. Dislocation-induced stop-and-go kinetics of interfacial transformations. Nature 607, 708–713 (2022).
Du, K. et al. Interface engineering breaks both stability and activity limits of RuO2 for sustainable water oxidation. Nat. Commun. 13, 5448 (2022).
Zhang, H. et al. Atomically engineered interfaces yield extraordinary electrostriction. Nature 609, 695–700 (2022).
Gong, Y. et al. Elemental de-mixing-induced epitaxial kesterite/CdS interface enabling 13%-efficiency kesterite solar cells. Nat. Energy 7, 966–977 (2022).
Chen, K. et al. Graphene oxide bulk material reinforced by heterophase platelets with multiscale interface crosslinking. Nat. Mater. 21, 1121–1129 (2022).
Su, C. et al. Tuning colour centres at a twisted hexagonal boron nitride interface. Nat. Mater. 21, 896–902 (2022).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Huang, X. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Zhou, M., Li, C. & Fang, J. Noble-metal based random alloy and intermetallic nanocrystals: syntheses and applications. Chem Rev. 121, 736–795 (2021).
Furukawa, S. & Komatsu, T. Intermetallic compounds: promising inorganic materials for well-structured and electronically modified reaction environments for efficient catalysis. ACS Catal. 7, 735–765 (2017).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Ryoo, R. et al. Rare-earth–platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Liu, Y. et al. Two-dimensional intermetallic PtBi/Pt core/shell nanoplates overcome tumor hypoxia for enhanced cancer therapy. Nanoscale 13, 14245–14253 (2021).
Yang, C.-L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Qiu, Y. et al. Construction of Pd–Zn dual sites to enhance the performance for ethanol electro-oxidation reaction. Nat. Commun. 12, 5273 (2021).
Maligal-Ganesh, R. V. et al. A ship-in-a-bottle strategy to synthesize encapsulated intermetallic nanoparticle catalysts: exemplified for furfural hydrogenation. ACS Catal. 6, 1754–1763 (2016).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Choi, D. S., Robertson, A. W., Warner, J. H., Kim, S. O. & Kim, H. Low-temperature chemical vapor deposition synthesis of Pt–Co alloyed nanoparticles with enhanced oxygen reduction reaction catalysis. Adv. Mater. 28, 7115–7122 (2016).
Cable, R. E. & Schaak, R. E. Reacting the unreactive: a toolbox of low-temperature solution-mediated reactions for the facile interconversion of nanocrystalline intermetallic compounds. J. Am. Chem. Soc. 128, 9588–9589 (2006).
Clarysse, J., Moser, A., Yarema, O., Wood, V. & Yarema, M. Size- and composition-controlled intermetallic nanocrystals via amalgamation seeded growth. Sci. Adv. 7, eabg1934 (2021).
Yao, Y. et al. High-entropy nanoparticles: synthesis–structure–property relationships and data-driven discovery. Science 376, eabn3103 (2022).
Chen, A. N. et al. Galvanic replacement of intermetallic nanocrystals as a route toward complex heterostructures. Nanoscale 13, 2618–2625 (2021).
Yu, J. et al. Synthesis of PtSn4 intermetallic nanodisks through a galvanic replacement mechanism. Chem. Mater. 34, 6968–6976 (2022).
Yu, J. et al. Precisely controlled synthesis of hybrid intermetallic–metal nanoparticles for nitrate electroreduction. ACS Appl. Mater. Interfaces 13, 52073–52081 (2021).
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Chee, S. W., Tan, S. F., Baraissov, Z., Bosman, M. & Mirsaidov, U. Direct observation of the nanoscale Kirkendall effect during galvanic replacement reactions. Nat Commun. 8, 1224 (2017).
Castilla-Amorós, L., Stoian, D., Pankhurst, J. R., Varandili, S. B. & Buonsanti, R. Exploring the chemical reactivity of gallium liquid metal nanoparticles in galvanic replacement. J. Am. Chem. Soc. 142, 19283–19290 (2020).
Xia, X., Wang, Y., Ruditskiy, A. & Xia, Y. 25th anniversary article: galvanic replacement: a simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 25, 6313–6333 (2013).
Oh, M. H. et al. Galvanic replacement reactions in metal oxide nanocrystals. Science 340, 964–968 (2013).
Zhu, Y. et al. Inverse iron oxide/metal catalysts from galvanic replacement. Nat. Commun. 11, 3269 (2020).
Wu, Y. et al. Dirac node arcs in PtSn4. Nat. Phys. 12, 667–671 (2016).
Chen, P.-C. et al. Interface and heterostructure design in polyelemental nanoparticles. Science 363, 959–964 (2019).
Kravchyk, K. et al. Monodisperse and inorganically capped Sn and Sn/SnO2 nanocrystals for high-performance Li-ion battery anodes. J. Am. Chem. Soc. 135, 4199–4202 (2013).
He, M., Protesescu, L., Caputo, R., Krumeich, F. & Kovalenko, M. V. A general synthesis strategy for monodisperse metallic and metalloid nanoparticles (In, Ga, Bi, Sb, Zn, Cu, Sn, and their alloys) via in situ formed metal long-chain amides. Chem. Mater. 27, 635–647 (2015).
Acknowledgements
J.Y. and W.H. acknowledge support from National Science Foundation grant CHE-2108307 and Iowa State University Trapp Award. J.Y. thanks R. Angelici for valuable discussions and suggestions on the manuscript. We thank K. Kovnir and J. Zaikina for valuable discussions and suggestions on the X-ray diffraction characterization.
Author information
Authors and Affiliations
Contributions
J.Y., Y.Y. and W.H. designed the conceptualization, methodology and visualization of this project. J.Y. performed all the experiments and drafted the original manuscript under the supervision of W.H. J.Y., Y.Y. and W.H. revised and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Ya-Wen Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single composition NMs prepared from Sn NPs.
(a) transmission electron microscopy (TEM) image of Sn NPs. High-angle annual dark-field-scanning TEM (HAADF-STEM) energy dispersive X-ray spectroscopy (EDS) mapping of (b) PtSn4, (c) PdSn4, (d) RhSn4, (e) AuSn, (f) RuxSny, (g) CoxSny, (h) Ni3Sn4, and (i) Cu6Sn5. Based on high resolution STEM images, RuxSny and CoxSny nanoparticles have Ru3Sn7 and CoSn2 intermetallic phases, respectively. Color of elements: Pt-red, Pd-cyan, Rh-purple, Au-yellow, Ru-green, Co-pink, Ni-jungle green, Cu-indigo, Sn-grey.
Extended Data Fig. 2 Crystal structure analysis of heterostructured iNM.
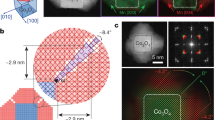
(a) High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images of PtSn4-PdSn4 iNMs, (b) the corresponding fast Fourier transform (FFT) of (a); the single set of diffraction pattern from FFT indicates the small lattice mismatch between PtSn4 and PdSn4; (d) STEM image of PtSn4-AuSn iNMs, (e) showing the FFT of PtSn4-AuSn iNMs; the dual diffraction pattern from FFT indicates the different lattice structure of PtSn4 and AuSn (pink: PtSn4 pattern; yellow: AuSn pattern; brown: overlap); (c) and (f) are schematic illustrations of PtSn4-PdSn4 iNM and PtSn4-AuSn iNM, respectively. Scale bar of (a) and (d): 5 nm.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–87 and Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, J., Yin, Y. & Huang, W. Engineered interfaces for heterostructured intermetallic nanomaterials. Nat. Synth 2, 749–756 (2023). https://doi.org/10.1038/s44160-023-00289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00289-4
This article is cited by
-
Stress-induced ordering evolution of 1D segmented heteronanostructures and their chemical post-transformations
Nature Communications (2024)
-
Interfaced intermetallics on the nanoscale
Nature Synthesis (2023)