Abstract
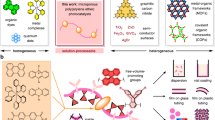
Fluoroalkenes are feedstocks in the manufacture of high-performance fluoropolymers with various applications. However, the synthesis of well-defined fluoropolymers typically requires harsh conditions and high-pressure techniques to handle gaseous fluoroalkenes. Here, we report the combination of a redox-relay pathway and thermally activated delayed fluorescence (TADF) catalysis to enable controlled copolymerizations of various fluoroalkenes, under ambient conditions. Using this method, a broad scope of main-chain fluoropolymers is prepared with excellent selectivity at low organocatalyst dosages (loadings down to 5 ppm). In addition, polymers with various sequences (for example, diblock and triblock) and topologies (either brush or branched) are synthesized by integration with different agents and synthetic protocols. Mechanistic studies reveal that the rationally designed organic TADF catalyst displays the unique characteristics of redox-relay-based electron transfer, a long lifetime of delayed fluorescence and fine-tuned electronic properties. This work provides an informative concept for precise polymer synthesis and may inspire advanced applications in fluoropolymer engineering.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information. Details about materials, analytic methods, experimental procedures, mechanistic studies, characterization data and NMR spectra are available in the Supplementary Information.
References
Nesvadba, P. in Encyclopedia of Radicals in Chemistry, Biology and Materials (Wiley, 2012).
Scheirs, J. Modern Fluoropolymers: High Performance Polymers for Diverse Applications (Wiley, 1997).
Ebnesajjad, S. Introduction to Fluoropolymers: Materials, Technology, and Applications (Elsevier, 2013).
Lv, J. & Cheng, Y. Fluoropolymers in biomedical applications: state-of-the-art and future perspectives. Chem. Soc. Rev. 50, 5435–5467 (2021).
Mohammad, S. A., Shingdilwar, S., Banerjee, S. & Améduri, B. Macromolecular engineering approach for the preparation of new architectures from fluorinated olefins and their applications. Prog. Polym. Sci. 106, 101255 (2020).
Pester, C. W. et al. Engineering surfaces through sequential stop-flow photopatterning. Adv. Mater. 28, 9292–9300 (2016).
Reis, M. et al. Machine-learning-guided discovery of 19F MRI agents enabled by automated copolymer synthesis. J. Am. Chem. Soc. 143, 17677–17689 (2021).
Ma, M. et al. Designing weakly solvating solid main-chain fluoropolymer electrolytes: synergistically enhancing stability toward Li anodes and high-voltage cathodes. ACS Energy Lett. 6, 4255–4264 (2021).
Moad, G., Rizzardo, E. & Thang, S. H. Living radical polymerization by the RAFT process. Aust. J. Chem. 58, 379–410 (2005).
Kamigaito, M., Ando, T. & Sawamoto, M. Metal-catalyzed living radical polymerization. Chem. Rev. 101, 3689–3745 (2001).
Matyjaszewski, K. & Tsarevsky, N. V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 136, 6513–6533 (2014).
Corrigan, N. et al. Reversible-deactivation radical polymerization (controlled/living radical polymerization): from discovery to materials design and applications. Prog. Polym. Sci. 111, 101311 (2020).
Boschet, F. & Améduri, B. (Co)polymers of chlorotrifluoroethylene: synthesis, properties, and applications. Chem. Rev. 114, 927–980 (2014).
Hansen, N. M. L., Jankova, K. & Hvilsted, S. Fluoropolymer materials and architectures prepared by controlled radical polymerizations. Eur. Polym. J. 43, 255–293 (2007).
Lee, Y., Boyer, C. & Kwon, M. S. Visible-light-driven polymerization towards the green synthesis of plastics. Nat. Rev. Mater. 7, 74–75 (2021).
Shanmugam, S. & Boyer, C. Organic photocatalysts for cleaner polymer synthesis. Science 352, 1053–1054 (2016).
Fors, B. P. & Hawker, C. J. Control of a living radical polymerization of methacrylates by light. Angew. Chem. Int. Ed. 51, 8850–8853 (2012).
Xu, J., Jung, K., Atme, A., Shanmugam, S. & Boyer, C. A robust and versatile photoinduced living polymerization of conjugated and unconjugated monomers and its oxygen tolerance. J. Am. Chem. Soc. 136, 5508–5519 (2014).
Theriot, J. C. et al. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 352, 1082–1086 (2016).
Pearson, R. M., Lim, C.-H., McCarthy, B. G., Musgrave, C. B. & Miyake, G. M. Organocatalyzed atom transfer radical polymerization using N-aryl phenoxazines as photoredox catalysts. J. Am. Chem. Soc. 138, 11399–11407 (2016).
Singh, V. K. et al. Highly efficient organic photocatalysts discovered via a computer-aided-design strategy for visible-light-driven atom transfer radical polymerization. Nat. Catal. 1, 794–804 (2018).
Kostov, G. et al. First amphiphilic poly(vinylidene fluoride-co-3,3,3-trifluoropropene)-b-oligo(vinyl alcohol) block copolymers as potential nonpersistent fluorosurfactants from radical polymerization controlled by xanthate. Macromolecules 44, 1841–1855 (2011).
Patil, Y. & Ameduri, B. First RAFT/MADIX radical copolymerization of tert-butyl 2-trifluoromethacrylate with vinylidene fluoride controlled by xanthate. Polym. Chem. 4, 2783–2799 (2013).
Tezuka, Y. & Oike, H. Topological polymer chemistry. Prog. Polym. Sci. 27, 1069–1122 (2002).
Cao, M., Liu, Y., Zhang, X., Li, F. & Zhong, M. Expanding the toolbox of controlled/living branching radical polymerization through simulation-informed reaction design. Chem 8, 1460–1475 (2022).
Corbin, D. A. & Miyake, G. M. Photoinduced organocatalyzed atom transfer radical polymerization (O-ATRP): precision polymer synthesis using organic photoredox catalysis. Chem. Rev. 122, 1830–1874 (2022).
Bryden, M. A. & Zysman-Colman, E. Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 50, 7587–7680 (2021).
Sharma, P. P. et al. Acid resistant PVDF-co-HFP based copolymer proton exchange membrane for electro-chemical application. J. Membr. Sci. 573, 485–492 (2019).
Quan, Q., Ma, M., Wang, Z., Gu, Y. & Chen, M. Visible-light-enabled organocatalyzed controlled alternating terpolymerization of perfluorinated vinyl ethers. Angew. Chem. Int. Ed. 60, 20443–20451 (2021).
Xu, J., Shanmugam, S., Duong, H. T. & Boyer, C. Organo-photocatalysts for photoinduced electron transfer-reversible addition–fragmentation chain transfer (PET-RAFT) polymerization. Polym. Chem. 6, 5615–5624 (2015).
Liu, X., Zhang, L., Cheng, Z. & Zhu, X. Metal-free photoinduced electron transfer–atom transfer radical polymerization (PET–ATRP) via a visible light organic photocatalyst. Polym. Chem. 7, 689–700 (2016).
Kutahya, C., Aykac, F. S., Yilmaz, G. & Yagci, Y. LED and visible light-induced metal free ATRP using reducible dyes in the presence of amines. Polym. Chem. 7, 6094–6098 (2016).
Treat, N. J. et al. Metal-free atom transfer radical polymerization. J. Am. Chem. Soc. 136, 16096–16101 (2014).
Chen, K., Zhou, Y., Han, S., Liu, Y. & Chen, M. Main-chain fluoropolymers with alternating sequence control via light-driven reversible-deactivation copolymerization in batch and flow. Angew. Chem. Int. Ed. 61, e202116135 (2022).
Chen, M., Zhong, M. & Johnson, J. A. Light-controlled radical polymerization: mechanisms, methods, and applications. Chem. Rev. 116, 10167–10211 (2016).
Lim, C.-H. et al. Intramolecular charge transfer and ion pairing in N,N-diaryl dihydrophenazine photoredox catalysts for efficient organocatalyzed atom transfer radical polymerization. J. Am. Chem. Soc. 139, 348–355 (2017).
Terenziani, F., Painelli, A., Katan, C., Charlot, M. & Blanchard-Desce, M. Charge instability in quadrupolar chromophores: symmetry breaking and solvatochromism. J. Am. Chem. Soc. 128, 15742–15755 (2006).
Speckmeier, E., Fischer, T. G. & Zeitler, K. A toolbox approach to construct broadly applicable metal-free catalysts for photoredox chemistry: deliberate tuning of redox potentials and importance of halogens in donor–acceptor cyanoarenes. J. Am. Chem. Soc. 140, 15353–15365 (2018).
Michaudel, Q. et al. Mechanistic insight into the photocontrolled cationic polymerization of vinyl ethers. J. Am. Chem. Soc. 139, 15530–15538 (2017).
Lakowicz, J. Principles of Fluorescence Spectroscopy (Springer, 2006).
Sugihara, S., Yoshida, A., Kono, T.-A., Takayama, T. & Maeda, Y. Controlled radical homopolymerization of representative cationically polymerizable vinyl ethers. J. Am. Chem. Soc. 141, 13954–13961 (2019).
Lee, K., Corrigan, N. & Boyer, C. Rapid high-resolution 3D printing and surface functionalization via type I photoinitiated RAFT polymerization. Angew. Chem. Int. Ed. 60, 8839–8850 (2021).
Narupai, B. et al. Simultaneous preparation of multiple polymer brushes under ambient conditions using microliter volumes. Angew. Chem. Int. Ed. 57, 13433–13438 (2018).
Baradie, B. & Shoichet, M. S. Synthesis of fluorocarbon-vinyl acetate copolymers in supercritical carbon dioxide: insight into bulk properties. Macromolecules 35, 3569–3575 (2002).
Perrier, S. 50th Anniversary perspective: RAFT polymerization-a user guide. Macromolecules 50, 7433–7447 (2017).
Friesen, C. M. & Améduri, B. Outstanding telechelic perfluoropolyalkylethers and applications therefrom. Prog. Polym. Sci. 81, 238–280 (2018).
Hawker, C. J., Frechet, J. M. J., Grubbs, R. B. & Dao, J. Preparation of hyperbranched and star polymers by a ‘living’, self-condensing free radical polymerization. J. Am. Chem. Soc. 117, 10763–10764 (1995).
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (NSFC, no. 21971044, M.C.), Shanghai Pilot Program for Basic Research—Fudan University 21TQ1400100 (no. 21TQ007, M.C.), Science and Technology Commission of Shanghai Municipality and State Key Laboratory of Molecular Engineering of Polymers.
Author information
Authors and Affiliations
Contributions
Y. Zhao and M.C. conceived and designed the research. Y. Zhao, Y.C. and K.C. performed synthesis. Y. Zhao, Y.C., H.Z. and Y. Zhou performed characterization. Y. Zhao, Y.C., H.Z., Y. Zhou, K.C., Y.G. and M.C. analysed data. Y. Zhao, Y.G. and M.C. wrote the manuscript and the supplemental information. All authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and methods, Figs. 1–125 and Tables 1–23.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Chen, Y., Zhou, H. et al. Controlled radical copolymerization of fluoroalkenes by using light-driven redox-relay catalysis. Nat. Synth 2, 653–662 (2023). https://doi.org/10.1038/s44160-023-00284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00284-9
This article is cited by
-
Ultraviolet light blocking optically clear adhesives for foldable displays via highly efficient visible-light curing
Nature Communications (2024)
-
Persistent radical anion of perylene dianhydride: an emerging metal-free photocatalyst for near-infrared photocontrolled RAFT polymerization
Science China Chemistry (2024)
-
Sequencing polymers to enable solid-state lithium batteries
Nature Materials (2023)
-
Continuous Synthesis of Main-Chain-type Fluorinated Graft Copolymers via Successive Flow START Polymerization and Cu(0)-Mediated RDRP
Chinese Journal of Polymer Science (2023)
-
Recent advances in fluorinated polymers: synthesis and diverse applications
Science China Chemistry (2023)