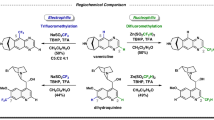
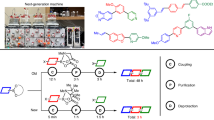
Abstract
Benzylic carbocations bearing an ortho- or para-hydroxyl group can be stabilized by forming quinone methides, which have been explored in enantioselective synthesis. However, those with a meta-hydroxyl group have remained almost unexplored in organic synthesis. The lack of resonance stabilization by a typical quinone methide form renders them not only difficult to generate, but also challenging to control for asymmetric bond formation. Here we report an efficient catalytic enantioselective reaction between meta-hydroxyl triarylmethanols and indoles, via triaryl carbocations, for the synthesis of tetraarylmethanes with excellent enantiocontrol. Control experiments reveal that the meta-hydroxyl group is essential for both reactivity and stereocontrol. Ortho-directing groups (alkoxyl, sulfenyl or fluoro) benefit enantiocontrol through secondary hydrogen-bonding interactions, but are not required for reactivity. The resulting tetraarylmethane products show anticancer activities, through a mechanism distinct from that of classical anticancer drugs.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source Data are provided with this Article. All data generated and analysed during this study are included in this Article, its Supplementary Information and its Source Data. The X-ray crystallographic coordinates of the structure of 8 (a derivative of 3p) have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2142024 and can be obtained free of charge from the CCDC via https://www.ccdc.cam.ac.uk/structures/.
References
Rokita, S. E. (ed.) Quinone Methides (Wiley, 2009).
Toteva, M. M. & Richard, J. P. The generation and reactions of quinone methides. Adv. Phys. Org. Chem. 45, 39–91 (2011).
Van De Water, R. W. & Pettus, T. R. J. T. o-Quinone methides: intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron 58, 5367–5406 (2002).
Li, Q. et al. Ortho-quinone methide finds its application in bioorthogonal ligation. Curr. Org. Chem. 18, 86–92 (2014).
Gnaim, S. & Shabat, D. Quinone-methide species, a gateway to functional molecular systems: from self-immolative dendrimers to long-wavelength fluorescent dyes. Acc. Chem. Res. 47, 2970–2984 (2014).
Pathak, T. P. & Sigman, M. S. Applications of ortho-quinone methide intermediates in catalysis and asymmetric synthesis. J. Org. Chem. 76, 9210–9215 (2011).
Wang, Z. & Sun, J. Recent advances in catalytic asymmetric reactions of o-quinone methides. Synthesis 47, 3629–3644 (2015).
Jaworski, A. A. & Scheidt, K. A. Emerging roles of in situ generated quinone methides in metal-free catalysis. J. Org. Chem. 81, 10145–10153 (2016).
Dorsch, C. & Schneider, C. Asymmetric Brønsted acid catalyzed cycloadditions of ortho-quinone methides and related compounds. Synthesis 54, 3125–3141 (2022).
Li, X., Li, Z. & Sun, J. Quinone methides and indole imine methides as intermediates in enantioselective catalysis. Nat. Synth. 1, 426–438 (2022).
Li, W., Xu, X., Zhang, P. & Li, P. Recent advances in the catalytic enantioselective reactions of para-quinone methides. Chem. Asian J. 13, 2350–2359 (2018).
El-Sepelgy, O., Haseloff, S., Alamsetti, S. K. & Schneider, C. Brønsted acid catalyzed, conjugate addition of β-dicarbonyls to in situ generated ortho-quinone methides—enantioselective synthesis of 4-aryl-4H-chromenes. Angew. Chem. Int. Ed. Engl. 53, 7923–7927 (2014).
Hsiao, C.-C., Liao, H.-H. & Rueping, M. Enantio- and diastereoselective access to distant stereocenters embedded within tetrahydroxanthenes: utilizing ortho-quinone methides as reactive intermediates in asymmetric Bronsted acid catalysis. Angew. Chem. Int. Ed. Engl. 53, 13258–13263 (2014).
Zhao, W., Wang, Z., Chu, B. & Sun, J. Enantioselective formation of all-carbon quaternary stereocenters from indoles and tertiary alcohols bearing a directing group. Angew. Chem. Int. Ed. Engl. 54, 1910–1913 (2015).
Tsui, G. C., Liu, L. & List, B. The organocatalytic asymmetric Prins cyclization. Angew. Chem. Int. Ed. Engl. 54, 7703–7706 (2015).
Wang, Z. et al. Organocatalytic asymmetric synthesis of 1,1-diarylethanes by transfer hydrogenation. J. Am. Chem. Soc. 137, 383–389 (2015).
Zhao, J.-J., Sun, S.-B., He, S.-H., Wu, Q. & Shi, F. Catalytic asymmetric inverse-electron-demand oxa-Diels–Alder reaction of in situ generated ortho-quinone methides with 3-methyl-2-vinylindoles. Angew. Chem. Int. Ed. Engl. 54, 5460–5464 (2015).
Lin, J.-S. et al. Cu/chiral phosphoric acid-catalyzed asymmetric three-component radical-initiated 1,2-dicarbofunctionalization of alkenes. J. Am. Chem. Soc. 141, 1074–1083 (2019).
Wang, Z., Wong, Y. F. & Sun, J. Catalytic asymmetric 1,6-conjugate addition of para-quinone methides: formation of all-carbon quaternary stereocenters. Angew. Chem. Int. Ed. Engl. 54, 13711–13714 (2015).
Qian, D., Wu, L., Lin, Z. & Sun, J. Organocatalytic synthesis of chiral tetrasubstituted allenes from racemic propargylic alcohols. Nat. Commun. 8, 567 (2017).
Ma, D., Miao, C.-B. & Sun, J. Catalytic enantioselective House–Meinwald rearrangement: efficient construction of all-carbon quaternary stereocenters. J. Am. Chem. Soc. 141, 13783–13787 (2019).
Wu, H., Wang, Q. & Zhu, J. Catalytic enantioselective pinacol and Meinwald rearrangements for the construction of quaternary stereocenters. J. Am. Chem. Soc. 141, 11372–11377 (2019).
Wang, Z., Zhu, Y., Pan, X., Wang, G. & Liu, L. Synthesis of chiral triarylmethanes bearing all-carbon quaternary stereocenters: catalytic asymmetric oxidative cross-coupling of 2,2-diarylacetonitriles and (hetero)arenes. Angew. Chem. Int. Ed. Engl. 59, 3053–3057 (2020).
Saha, S., Alamsetti, S. K. & Schneider, C. Chiral Brønsted acid-catalyzed Friedel–Crafts alkylation of electron-rich arenes with in situ-generated ortho-quinone methides: highly enantioselective synthesis of diarylindolylmethanes and triarylmethanes. Chem. Commun. 51, 1461–1464 (2015).
Sun, M. et al. Catalytic asymmetric (4+3) cyclizations of in situ generated ortho-quinone methides with 2-indolylmethanols. Angew. Chem. Int. Ed. Engl. 58, 8703–8708 (2019).
Jiang, F. et al. A strategy for synthesizing axially chiral naphthyl-indoles: catalytic asymmetric addition reactions of racemic substrates. Angew. Chem. Int. Ed. Engl. 58, 15104–15110 (2019).
Tsuji, N. et al. Activation of olefins via asymmetric Brønsted acid catalysis. Science 359, 1501–1505 (2018).
Mahlau, M. & List, B. Asymmetric counteranion-directed catalysis: concept, definition, and applications. Angew. Chem. Int. Ed. Engl. 52, 518–533 (2013).
Brak, K. & Jacobsen, E. N. Asymmetric ion-pairing catalysis. Angew. Chem. Int. Ed. Engl. 52, 534–561 (2013).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Properzi, R. et al. Catalytic enantiocontrol over a non-classical carbocation. Nat. Chem. 12, 1174–1179 (2020).
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. Engl. 43, 1566–1568 (2004).
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric binol-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation, Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Akiyama, T. & Mori, K. Stronger Brønsted acids: recent progress. Chem. Rev. 115, 9277–9306 (2015).
James, T., van Gemmeren, M. & List, B. Development and applications of disulfonimides in enantioselective organocatalysis. Chem. Rev. 115, 9388–9409 (2015).
Kikuchi, J. & Terada, M. Enantioconvergent substitution reactions of racemic electrophiles by organocatalysis. Chem. Eur. J. 27, 10215–10225 (2021).
Li, X. et al. Catalytic enantioselective synthesis of chiral tetraarylmethanes. Nat. Catal. 3, 1010–1019 (2020).
Li, Z. et al. Organocatalytic asymmetric formal oxidative coupling for the construction of all-aryl quaternary stereocenters. Chem. Sci. 12, 11793–11798 (2021).
Kochanowska-Karamyan, A. J. & Hamann, M. T. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 110, 4489–4497 (2010).
Chadha, N. & Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: bird’s eye view. Eur. J. Med. Chem. 134, 159–184 (2017).
Kashyap, M. et al. Indenoindolone derivatives as topoisomerase II-inhibiting anticancer agents. Bioorg. Med. Chem. Lett. 23, 934–938 (2013).
Nagaoka, M., Numazawa, S., Yokoyama, N., Kim, S. I. & Kusano, S. Indenoindole derivative and organic electroluminescent element. US patent 9985216B2 (2018).
Batlle, E. & Clevers, H. Cancer stem cells revisited. Nat. Med. 23, 1124–1134 (2017).
Berghe, T. V. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2010).
Su, Z., Yang, Z., Xie, L., DeWitt, J. & Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ. 23, 748–756 (2016).
Linkermann, A. & Green, D. R. Necroptosis. N. Engl. J. Med. 370, 455–465 (2014).
Suntharalingam, K. et al. Necroptosis-inducing rhenium(V) oxo complexes. J. Am. Chem. Soc. 137, 2967–2974 (2015).
Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (91956114, 22077108 and 21877092), the Science Technology and Innovation Committee of Shenzhen Municipality (JCYJ20200109141408054 and JCYJ20210324120004011), the Hong Kong Research Grants Council (16303420, 16309321, 11307419, 11303320 and 11302221) and the Innovation and Technology Commission (ITC-CNERC14SC01). We thank H. H. Y. Sung for help in structure elucidation by X-ray crystallography.
Author information
Authors and Affiliations
Contributions
X.T. and Q.W. performed the chemical syntheses and wrote the paper. Z.D. and S.C. performed the experiments for the biological study and wrote the paper. G.Z. directed the biological study and wrote the paper. J.S. conceived and directed the project and wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
X.T. and J.S. are owners of a Chinese patent application (202210842981.9). The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Luca Bernardi, Robert Musiol and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, characterization data and mechanistic studies, Supplementary Figs. 1–4, Tables 1–5, NMR spectra and HPLC traces.
Supplementary Data 1
Crystallographic data for 8; CCDC 2142024.
Source data
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, X., Deng, Z., Wang, Q. et al. Enantioselective synthesis of tetraarylmethanes through meta-hydroxyl-directed benzylic substitution. Nat. Synth 2, 275–285 (2023). https://doi.org/10.1038/s44160-022-00211-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00211-4