Abstract
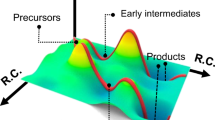
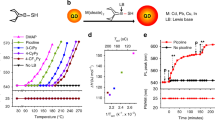
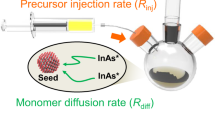
Large ZnSe nanocrystals are expected to be promising blue-light emitters with an emission peak of 455–475 nm, which is important for the construction of display apparatus. The final size of ZnSe nanocrystals via one-step injection can be varied by the reactivity of the Zn and Se precursors; however, it has a limit of <5 nm. To describe the key factors in determining the final size of ZnSe nanocrystals, we proposed a nuclei number-considered LaMer model based on the Maxwell–Boltzmann distribution of crystal embryos. As a result, a general strategy of reactivity-controlled epitaxial growth was developed to synthesize large ZnSe nanocrystals through sequential injection of high-reactivity and low-reactivity Zn and Se precursors. The resultant ZnSe nanocrystals achieved pure blue emission between 455 and 470 nm. We further fabricated stable, large ZnSe/ZnS core–shell nanocrystals with photoluminescence quantum yields up to approximately 60%. Moreover, the reactivity-controlled epitaxial growth strategy is versatile and could be used to synthesize large ZnSe, CdSe and PbSe nanocrystals with average sizes up to 35 nm, 76 nm and 87 nm, respectively. The control of quantum-confined and classical effects in these large semiconductor nanocrystals will open up new directions for fundamental research and application exploration.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files.
References
García de Arquer, F. P. et al. Semiconductor quantum dots: technological progress and future challenges. Science 373, eaaz8541 (2021).
Efros, A. L. & Brus, L. E. Nanocrystal quantum dots: from discovery to modern development. ACS Nano 15, 6192–6210 (2021).
Kim, T. et al. Efficient and stable blue quantum dot light-emitting diode. Nature 586, 385–389 (2020).
Hines, M. A. & Guyot-Sionnest, P. Bright UV-blue luminescent colloidal ZnSe nanocrystals. J. Phys. Chem. B 102, 3655–3657 (1998).
Li, L. S., Pradhan, N., Wang, Y. & Peng, X. High quality ZnSe and ZnS nanocrystals formed by activating zinc carboxylate precursors. Nano Lett. 4, 2261–2264 (2004).
Ji, B., Koley, S., Slobodkin, I., Remennik, S. & Banin, U. ZnSe/ZnS core/shell quantum dots with superior optical properties through thermodynamic shell growth. Nano Lett. 20, 2387–2395 (2020).
Gao, M. et al. Bulk-like ZnSe quantum dots enabling efficient ultranarrow blue light-emitting diodes. Nano Lett. 21, 7252–7260 (2021).
Murray, C., Norris, D. J. & Bawendi, M. G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Peng, X., Wickham, J. & Alivisatos, A. Kinetics of II-VI and III-V colloidal semiconductor nanocrystal growth: ‘focusing’ of size distributions. J. Am. Chem. Soc. 120, 5343–5344 (1998).
Peng, Z. A. & Peng, X. Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J. Am. Chem. Soc. 124, 3343–3353 (2002).
Park, J., Joo, J., Kwon, S. G., Jang, Y. & Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. 46, 4630–4660 (2007).
Owen, J. S., Chan, E. M., Liu, H. & Alivisatos, A. P. Precursor conversion kinetics and the nucleation of cadmium selenide nanocrystals. J. Am. Chem. Soc. 132, 18206–18213 (2010).
Lee, J., Yang, J., Kwon, S. G. & Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 1, 16034 (2016).
Liu, M. et al. Probing intermediates of the induction period prior to nucleation and growth of semiconductor quantum dots. Nat. Commun. 8, 15467 (2017).
Leffler, V., Ehlert, S., Förster, B., Dulle, M. & Förster, S. Nanoparticle heat-up synthesis: in situ X-ray diffraction and extension from classical to nonclassical nucleation and growth theory. ACS Nano 15, 840–856 (2021).
Qu, L., Peng, Z. A. & Peng, X. Alternative routes toward high quality CdSe nanocrystals. Nano Lett. 1, 333–337 (2001).
Talapin, D. V., Rogach, A. L., Kornowski, A., Haase, M. & Weller, H. Highly luminescent monodisperse CdSe and CdSe/ZnS nanocrystals synthesized in a hexadecylamine-trioctylphosphine oxide-trioctylphospine mixture. Nano Lett. 1, 207–211 (2001).
Chen, Y. et al. ‘Giant’ multishell CdSe nanocrystal quantum dots with suppressed blinking. J. Am. Chem. Soc. 130, 5026–5027 (2008).
Chen, O. et al. Compact high-quality CdSe-CdS core-shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 12, 445–451 (2013).
Manna, L., Scher, E. C. & Alivisatos, A. P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700–12706 (2000).
Liu, L. et al. Shape control of CdSe nanocrystals with zinc blende structure. J. Am. Chem. Soc. 131, 16423–16429 (2009).
Peng, X. et al. Shape control of CdSe nanocrystals. Nature 404, 59–61 (2000).
Ithurria, S. & Dubertret, B. Quasi 2D colloidal CdSe platelets with thicknesses controlled at the atomic level. J. Am. Chem. Soc. 130, 16504–16505 (2008).
Li, H., Kanaras, A. G. & Manna, L. Colloidal branched semiconductor nanocrystals: state of the art and perspectives. Acc. Chem. Res. 46, 1387–1396 (2013).
Jia, G. & Banin, U. A general strategy for synthesizing colloidal semiconductor zinc chalcogenide quantum rods. J. Am. Chem. Soc. 136, 11121–11127 (2014).
Ning, J., Liu, J., Levi-Kalisman, Y., Frenkel, A. I. & Banin, U. Controlling anisotropic growth of colloidal ZnSe nanostructures. J. Am. Chem. Soc. 140, 14627–14637 (2018).
Ji, B. et al. Strain-controlled shell morphology on quantum rods. Nat. Commun. 10, 2 (2019).
Cunningham, P. D., Coropceanu, I., Mulloy, K., Cho, W. & Talapin, D. V. Quantized reaction pathways for solution synthesis of colloidal ZnSe nanostructures: a connection between clusters, nanowires, and two-dimensional nanoplatelets. ACS nano 14, 3847–3857 (2020).
Acharya, S., Sarma, D., Jana, N. R. & Pradhan, N. An alternate route to high-quality ZnSe and Mn-doped ZnSe nanocrystals. J. Phys. Chem. Lett. 1, 485–488 (2010).
Jang, E.-P. et al. Synthesis of alloyed ZnSeTe quantum dots as bright, color-pure blue emitters. ACS Appl. Mater. Interfaces 11, 46062–46069 (2019).
Wei, S.-H., Zhang, S. & Zunger, A. First-principles calculation of band offsets, optical bowings, and defects in CdS, CdSe, CdTe, and their alloys. J. Appl. Phys. 87, 1304–1311 (2000).
Guo, Y., Alvarado, S. R., Barclay, J. D. & Vela, J. Shape-programmed nanofabrication: understanding the reactivity of dichalcogenide precursors. ACS Nano 7, 3616–3626 (2013).
Park, J., Jayaraman, A., Schrader, A. W., Hwang, G. W. & Han, H. S. Controllable modulation of precursor reactivity using chemical additives for systematic synthesis of high-quality quantum dots. Nat. Commun. 11, 5748 (2020).
Gary, D. C., Glassy, B. A. & Cossairt, B. M. Investigation of indium phosphide quantum dot nucleation and growth utilizing triarylsilylphosphine precursors. Chem. Mater. 26, 1734–1744 (2014).
Toufanian, R., Zhong, X., Kays, J. C., Saeboe, A. M. & Dennis, A. M. Correlating ZnSe quantum dot absorption with particle size and concentration. Chem. Mater. 33, 7527–7536 (2021).
Van Embden, J., Chesman, A. S. & Jasieniak, J. J. The heat-up synthesis of colloidal nanocrystals. Chem. Mater. 27, 2246–2285 (2015).
McMurtry, B. M. et al. Continuous nucleation and size dependent growth kinetics of indium phosphide nanocrystals. Chem. Mater. 32, 4358–4368 (2020).
Prins, P. T. et al. Extended nucleation and superfocusing in colloidal semiconductor nanocrystal synthesis. Nano Lett. 21, 2487–2496 (2021).
Talapin, D. V., Rogach, A. L., Haase, M. & Weller, H. Evolution of an ensemble of nanoparticles in a colloidal solution: theoretical study. J. Phys. Chem. B 105, 12278–12285 (2001).
Campos, M. P. et al. Growth kinetics determine the polydispersity and size of PbS and PbSe nanocrystals. Chem. Sci. 13, 4555–4565 (2022).
LaMer, V. K. & Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 72, 4847–4854 (1950).
Kashchiev, D. Nucleation (Elsevier, 2000).
Robb, D. T. & Privman, V. Model of nanocrystal formation in solution by burst nucleation and diffusional growth. Langmuir 24, 26–35 (2008).
Rempel, J. Y., Bawendi, M. G. & Jensen, K. F. Insights into the kinetics of semiconductor nanocrystal nucleation and growth. J. Am. Chem. Soc. 131, 4479–4489 (2009).
Thanh, N. T., Maclean, N. & Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 114, 7610–7630 (2014).
Abe, S., Capek, R. K., De Geyter, B. & Hens, Z. Tuning the postfocused size of colloidal nanocrystals by the reaction rate: from theory to application. ACS Nano 6, 42–53 (2012).
Sosso, G. C. et al. Crystal nucleation in liquids: open questions and future challenges in molecular dynamics simulations. Chem. Rev. 116, 7078–7116 (2016).
Leite, E. R. & Ribeiro, C. Crystallization and Growth of Colloidal Nanocrystals (Springer Science & Business Media, 2011).
Xie, R., Li, Z. & Peng, X. Nucleation kinetics vs chemical kinetics in the initial formation of semiconductor nanocrystals. J. Am. Chem. Soc. 131, 15457–15466 (2009).
Scholes, G. D. Controlling the optical properties of inorganic nanoparticles. Adv. Fun. Mater. 18, 1157–1172 (2008).
Sugimoto, T. Monodispersed Particles (Elsevier, 2019).
Carbone, L. et al. Synthesis and micrometer-scale assembly of colloidal CdSe/CdS nanorods prepared by a seeded growth approach. Nano Lett. 7, 2942–2950 (2007).
Reiss, P., Protiere, M. & Li, L. Core/shell semiconductor nanocrystals. Small 5, 154–168 (2009).
Franke, D. et al. Continuous injection synthesis of indium arsenide quantum dots emissive in the short-wavelength infrared. Nat. Commun. 7, 12749 (2016).
Riedinger, A. et al. An intrinsic growth instability in isotropic materials leads to quasi-two-dimensional nanoplatelets. Nat. Mater. 16, 743–748 (2017).
Acknowledgements
This work was supported by Beijing Natural Science Foundation (Z210018, H.Z.), National Natural Science Foundation of China (61735004, H.Z.) and BOE Technology Group Co., Ltd. We would like to thank the Experimental Center of Advanced Materials of Beijing Institute of Technology for the support in materials synthesis and characterization. Z.L. and R.L. acknowledge the support from the S&T Program of Hebei under grant (216Z0601G, R.L.). The authors would like to acknowledge H. Bao (Beijing Institute of Technology) for checking the calculation of the diffusion-controlled model.
Author information
Authors and Affiliations
Contributions
H.Z., Y.L. and Z.C. conceptualized the project. H.Z., R.L. and G.Y. supervised the project. Z.L., M.L. and K.G. performed the materials synthesis and conducted the characterization measurements. H.Z., Z.L. and X.W. proposed the nucleation model. Z.L., G.Y. and H.Z. analysed the results and wrote the draft of the manuscript with subsequent input of the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Zuliang Du, Guohua Jia and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. The primary handling editor was Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Figs. 1–25, Tables 1–2 and Sections 1–4.
Supplementary Data Fig. 1
Statistical Source Data for Supplementary Figs. 1–9, 11–15, 17–21, 24 and 25.
Supplementary Data Fig. 2
Unprocessed TEM and STEM images for Supplementary Figs. 1, 10, 11, 22 and 23.
Source data
Source Data Fig. 1
Raw data of absorption and PL spectra, and plots of PL peak, UV peak, FWHM and diameter.
Source Data Fig. 2
Raw data of the plots of absorbance and nanocrystal concentration at different reaction conditions. Raw data of plots of standard deviation and the simulation data. Calculation data of diffusion radius.
Source Data Fig. 3
Raw data of absorption and PL spectra of different growth processes of ZnSe nanocrystals.
Source Data Fig. 3
Unprocessed TEM images of ZnSe nanocrystals with different sizes.
Source Data Fig. 4
Raw data of absorption and PL spectra of ZnSe core and ZnSe/ZnS core–shell nanocrystals. Raw data of plots of PLQY, PL peak and FWHM. Raw data of XRD patterns for ZnSe core and ZnSe/ZnS core–shell nanocrystals.
Source Data Fig. 4
Unprocessed TEM images of ZnSe and ZnSe/ZnS nanocrystals as well as their corresponding HRTEM images.
Source Data Fig. 5
Unprocessed TEM images of CdSe and PbSe nanocrystals with different sizes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Long, Z., Liu, M., Wu, Xg. et al. A reactivity-controlled epitaxial growth strategy for synthesizing large nanocrystals. Nat. Synth 2, 296–304 (2023). https://doi.org/10.1038/s44160-022-00210-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00210-5