Abstract
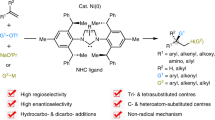
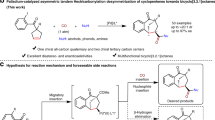
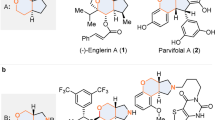
Development of enantioselective cycloaddition reactions involving three-carbon units (n + 3) is challenging because of the instability of the required zwitterionic three-carbon units. The reactivity of these zwitterionic species is particularly intriguing due to the prevalence of odd-numbered carbocyclic ring motifs in natural products. Although cycloadditions with 2-oxyallyl cations are generally well developed, cycloadditions are rare with 2-aminoallyl cations, with catalytic examples limited to intramolecular reactions involving furan derivatives. Here we report a method for copper-catalysed formation of 2-aminoallyl cations from ethynyl methylene cyclic carbamates and subsequent enantioselective (4 + 3)-cycloaddition reactions between 2-aminoallyl cations and dienol silyl ethers. The seven-membered ring carbocyclic products are formed in good yields and with high enantiocontrol. The synthetic utility of the reaction products has been demonstrated through a series of reductive, cross-coupling and cyclization transformations. Mechanistic studies reveal that the reaction involves a dinuclear copper catalyst and occurs stepwise, with formation of the second C–C bond as the turnover-limiting step.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The experimental data and the characterization data for all the compounds prepared during these studies are provided in the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2141995 (3ka), 2141996 (4aa), 2175552 (6a), 2175546 (7) and 2141998 ((L2-Cu)2(BF4)2). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Kobayashi, S. & Jorgensen, K. A. (eds) Cycloaddition Reactions in Organic Synthesis (Wiley, 2002).
Poplata, S., Troster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Crimmins, M. T. Synthetic applications of intramolecular enone–olefin photocycloadditions. Chem. Rev. 88, 1453–1473 (1988).
Sarkar, D., Bera, N. & Ghosh, S. [2 + 2] photochemical cycloaddition in organic synthesis. Eur. J. Org. Chem. 2020, 1310–1326 (2020).
Kagan, H. B. & Riant, O. Catalytic asymmetric Diels–Alder reactions. Chem. Rev. 92, 1007–1019 (1992).
Brieger, G. & Bennett, J. N. The intramolecular Diels–Alder reaction. Chem. Rev. 80, 63–97 (1980).
Pindur, U., Lutz, G. & Otto, C. Acceleration and selectivity enhancement of Diels–Alder reactions by special and catalytic methods. Chem. Rev. 93, 741–761 (1993).
Winkler, J. D. Tandem Diels–Alder cycloadditions in organic synthesis. Chem. Rev. 96, 167–176 (1996).
Nicolaou, K. C., Snyder, S. A., Montagnon, T. & Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1698 (2002).
Trost, B. M. & Lam, T. M. Development of diamidophosphite ligands and their application to the palladium-catalyzed vinyl-substituted trimethylenemethane asymmetric [3 + 2] cycloaddition. J. Am. Chem. Soc. 134, 11319–11321 (2012).
Trost, B. M., Morris, P. J. & Sprague, S. J. Palladium-catalyzed diastereo- and enantioselective formal [3 + 2]-cycloadditions of substituted vinylcyclopropanes. J. Am. Chem. Soc. 134, 17823–17831 (2012).
Trost, B. M., Ehmke, V., O’Keefe, B. M. & Bringley, D. A. Palladium-catalyzed dearomative trimethylenemethane cycloaddition reactions. J. Am. Chem. Soc. 136, 8213–8216 (2014).
Trost, B. M., Wang, Y. & Hung, C.-I. Use of α-trifluoromethyl carbanions for palladium-catalyzed asymmetric cycloadditions. Nat. Chem. 12, 294–301 (2020).
Cheng, Q., Xie, J.-H., Weng, Y.-C. & You, S.-L. Pd-catalyzed dearomatization of anthranils with vinylcyclopropanes by [4 + 3] cyclization reaction. Angew. Chem. Int. Ed. 58, 5739–5743 (2019).
Yang, L.-C. et al. Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions. Nat. Chem. 12, 860–868 (2020).
Hoffmann, H. M. R. Syntheses of seven- and five-membered rings from allyl cations. Angew. Chem. Int. Ed. 12, 819–835 (1973).
Noyori, R., Shimizu, F., Fukuta, K., Takaya, H. & Hayakawa, Y. Regioselectivity of the iron carbonyl promoted cyclocoupling reaction of α,α′-dibromo ketones with olefins and dienes. J. Am. Chem. Soc. 99, 5196–5198 (1977).
Takaya, H., Makino, S., Hayakawa, Y. & Noyori, R. Reactions of polybromo ketones with 1,3-dienes in the presence of iron carbonyls. New 3 + 4 → 7 cyclocoupling reaction forming 4-cycloheptenones. J. Am. Chem. Soc. 100, 1765–1777 (1978).
Yin, Z., He, Y. & Chiu, P. Application of (4 + 3) cycloaddition strategies in the synthesis of natural products. Chem. Soc. Rev. 47, 8881–8924 (2018).
Masuya, K., Domon, K., Tanino, K. & Kuwajima, I. Highly regio- and stereoselective [3+2] cyclopentanone annulation using a 3-(alkylthio)-2-siloxyallyl cationic species. J. Am. Chem. Soc. 120, 1724–1731 (1998).
Krenske, E. H. et al. Intramolecular oxyallyl–carbonyl (3 + 2) cycloadditions. J. Am. Chem. Soc. 135, 5242–5245 (2013).
Li, H., Hughes, R. P. & Wu, J. Dearomative indole (3 + 2) cycloaddition reactions. J. Am. Chem. Soc. 136, 6288–6296 (2014).
Hoffmann, H. M. R. The cycloaddition of allyl cations to 1,3-dienes: general method for the synthesis of seven-membered carbocycles. New synthetic methods. Angew. Chem. Int. Ed. 23, 1–19 (1984).
Harmata, M. Exploration of fundamental and synthetic aspects of the intramolecular 4 + 3 cycloaddition reaction. Acc. Chem. Res. 34, 595–605 (2001).
Harmata, M., Elomari, S. & Barnes, C. L. Intramolecular 4 + 3 cycloadditions. Cycloaddition reactions of cyclic alkoxyallylic and oxyallylic cations. J. Am. Chem. Soc. 118, 2860–2871 (1996).
Xiong, H., Hsung, R. P., Berry, C. R. & Rameshkumar, C. The first epoxidations of 1-amidoallenes. A general entry to nitrogen-substituted oxyallyl cations in highly stereoselective [4 + 3] cycloadditions. J. Am. Chem. Soc. 123, 7174–7175 (2001).
Rameshkumar, C. & Hsung, R. P. A tandem epoxidation/stereoselective intramolecular [4 + 3] cycloaddition reaction involving nitrogen-stabilized oxyallyl cations derived from chiral allenamides. Angew. Chem. Int. Ed. 43, 615–618 (2004).
Chung, W. K. et al. Inter- and intramolecular [4 + 3] cycloadditions using epoxy enol silanes as functionalized oxyallyl cation precursors. J. Am. Chem. Soc. 131, 4556–4557 (2009).
Antoline, J. E., Krenske, E. H., Lohse, A. G., Houk, K. N. & Hsung, R. P. Stereoselectivities and regioselectivities of (4 + 3) cycloadditions between allenamide-derived chiral oxazolidinone-stabilized oxyallyls and furans: experiment and theory. J. Am. Chem. Soc. 133, 14443–14451 (2011).
Lo, B., Lam, S., Wong, W.-T. & Chiu, P. Asymmetric (4 + 3) cycloadditions of enantiomerically enriched epoxy enolsilanes. Angew. Chem. Int. Ed. 51, 12120–12123 (2012).
Fu, C. et al. (4+3) Cycloaddition reactions of N-alkyl oxidopyridinium ions. Angew. Chem. Int. Ed. 56, 14682–14687 (2017).
Harmata, M., Ghosh, S. K., Hong, X., Wacharasindhu, S. & Kirchhoefer, P. Asymmetric organocatalysis of 4 + 3 cycloaddition reactions. J. Am. Chem. Soc. 125, 2058–2059 (2003).
Huang, J. & Hsung, R. P. Chiral Lewis acid-catalyzed highly enantioselective [4 + 3] cycloaddition reactions of nitrogen-stabilized oxyallyl cations derived from allenamides. J. Am. Chem. Soc. 127, 50–51 (2005).
Villar, L. et al. Enantioselective oxidative (4 + 3) cycloadditions between allenamides and furans through bifunctional hydrogen-bonding/ion-pairing interactions. Angew. Chem. Int. Ed. 56, 10535–10538 (2017).
Banik, S. M., Levina, A., Hyde, A. M. & Jacobsen, E. N. Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 358, 761–764 (2017).
Schmid, R. & Schmid, H. Silberioneninduzierte Reaktion von 3-Chlor-2-pyrrolidinocyclohexen mit 1,3-Dienen. Helv. Chim. Acta 57, 1883–1886 (1974).
Kende, A. S. & Huang, H. Asymmetric [4 + 3] cycloadditions from chiral α-chloro imines. Tetrahedron Lett. 38, 3353–3356 (1997).
Lee, J. et al. Fragmentation of alkoxy radicals and oxidative elimination of alicyclic iodides. J. Org. Chem. 59, 6955–6964 (1994).
Kim, H., Ziani-Cherif, C., Oh, J., Lee, D. & Cha, J. K. New [4 + 3] cycloaddition approach to cis-2,8-disubstituted oxocanes. J. Org. Chem. 60, 792–793 (1995).
Prié, G. et al. A Lewis acid catalyzed intramolecular [4 + 3] cycloaddition route to polycyclic systems that contain a seven-membered ring. Angew. Chem. Int. Ed. 43, 6517–6519 (2004).
Trost, B. M., Huang, Z. & Murhade, G. M. Catalytic palladium–oxyallyl cycloaddition. Science 362, 564–568 (2018).
Zou, Y., Chen, S. & Houk, K. N. Origins of selective formation of 5‑vinyl-2-methylene furans from oxyallyl/diene (3 + 2) cycloadditions with Pd(0) catalysis. J. Am. Chem. Soc. 141, 12382–12387 (2019).
Trost, B. M. & Huang, Z. Catalytic (3 + 2) palladium–aminoallyl cycloaddition with conjugated dienes. Angew. Chem. Int. Ed. 58, 6396–6499 (2019).
Chai, W. et al. Lewis acid-promoted ligand-controlled regiodivergent cycloaddition of Pd-oxyallyl with 1,3-dienes: reaction development and origins of selectivities. J. Am. Chem. Soc. 143, 3595–3603 (2021).
Zheng, Y., Qin, T. & Zi, W. Enantioselective inverse electron-demand (3 + 2) cycloaddition of palladium–oxyallyl enabled by a hydrogen-bond-donating ligand. J. Am. Chem. Soc. 143, 1038–1045 (2021).
Miyake, Y., Uemura, S. & Nishibayashi, Y. Catalytic propargylic substitution reactions. ChemCatChem 1, 342–356 (2009).
Roy, R. & Saha, S. Scope and advances in the catalytic propargylic substitution reaction. RSC Adv. 8, 31129–31193 (2018).
Ding, C.-H. & Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Zhang, D.-Y. & Hu, X.-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 56, 283–295 (2015).
Berger, K. J. et al. Direct deamination of primary amines via isodiazene intermediates. J. Am. Chem. Soc. 143, 17366–17373 (2021).
Li, R.-Z., Liu, D.-Q. & Niu, D. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 3, 672–680 (2020).
Nakajima, K., Shibata, M. & Nishibayashi, Y. Copper-catalyzed enantioselective propargylic etherifification of propargylic esters with alcohols. J. Am. Chem. Soc. 137, 2472–2475 (2015).
Hu, X.-H., Liu, Z.-T., Shao, L. & Hu, X.-P. Recent advances in catalytic stereo-controlled cycloaddition with terminal propargylic compounds. Synthesis 47, 913–923 (2015).
Zhang, C. et al. Highly diastereo-/enantioselective Cu-catalyzed [3 + 3] Cycloaddition of propargyl esters with cyclic enamines toward chiral bicyclo[n.3.1] frameworks. J. Am. Chem. Soc. 134, 9585–9588 (2012).
Zhu, F.-L., Wang, Y.-H., Zhang, D.-Y., Xu, J. & Hu, X.-P. Enantioselective synthesis of highly functionalized dihydrofurans via copper-catalyzed asymmetric formal [3 + 2] cycloaddition of β-ketoesters with propargylic esters with β-keto acids. Angew. Chem. Int. Ed. 53, 10223–10227 (2014).
Shao, L., Wang, Y.-H., Zhang, D.-Y., Xu, J. & Hu, X.-P. Desilylation-activated propargylic transformation: enantioselective Cu-catalyzed [3 + 2] cycloaddition of propargylic esters with β-naphthol or phenol derivatives. Angew. Chem. Int. Ed. 55, 5014–5018 (2016).
Wang, Q. et al. Catalytic asymmetric [4 + 1] annulation of sulfur ylides with copper–allenylidene intermediates. J. Am. Chem. Soc. 138, 8360–8363 (2016).
Song, J., Zhang, Z.-J. & Gong, L.-Z. Asymmetric [4 + 2] annulation of C1 ammonium enolates with copper–allenylidenes. Angew. Chem. Int. Ed. 56, 5212–5216 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (numbers 21871150, 22071118 and 22188101 for W. Z.) and the Fundamental Research Funds for Central University. We thank the Haihe Laboratory of Sustainable Chemical Transformations for financial support.
Author information
Authors and Affiliations
Contributions
W.Z. conceived and supervised this work. L.S. conducted studies and prepared the Supplementary Information. Z.L. and B.G. synthesized some substrates and catalysts. All the authors co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Pauline Chiu, Mike Shipman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary sections 1–10.
Supplementary Data 1
Crystallographic data for 3ka CCDC 2141995.
Supplementary Data 2
Crystallographic data for 4aa CCDC 2141996.
Supplementary Data 3
Structure factors file for compound 4aa CCDC 2141996.
Supplementary Data 4
Crystallographic data for 6a CCDC 2175552.
Supplementary Data 5
Crystallographic data for 7 CCDC 2175546.
Supplementary Data 6
Crystallographic data for (L2-Cu)2(BF4)2 CCDC 2141998.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, L., Lin, Z., Guo, B. et al. Synthesis of cycloheptanoids through catalytic enantioselective (4 + 3)-cycloadditions of 2-aminoallyl cations with dienol silyl ethers. Nat. Synth 1, 883–891 (2022). https://doi.org/10.1038/s44160-022-00150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00150-0