Abstract
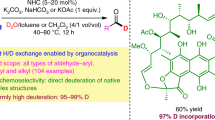
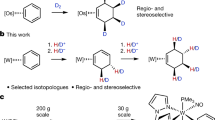
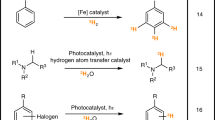
Efficient and practical construction of deuterated molecules has been a long-standing challenge. Although α-deutero carboxylic acids are ubiquitous structural components that serve as a wide array of synthons, and are thus in high demand, α-selective and mild methods for the deuteration of carboxylic acids have remained unexplored. Here we report the development of a ternary catalytic system for the α-deuteration of carboxylic acids in high yields with excellent levels of α-deuteration. The method shows wide functional group tolerance, including the late-stage deuteration of complex molecules, pharmaceuticals and natural products. Mechanistic studies and pKa calculations indicate that the reaction proceeds through the enolization of an acyl pyridinium species. The process was applied to the synthesis of a deuterated EP3 receptor antagonist, with the deutero analogue showing increased metabolic stability in human microsomes compared with the proto compound.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available within the article and its Supplementary Information.
References
Gant, T. G. Using deuterium in drug discovery: leaving the label in the drug. J. Med. Chem. 57, 3595–3611 (2014).
Pirali, T., Serafini, M., Cargnin, S. & Genazzani, A. A. Applications of deuterium in medicinal chemistry. J. Med. Chem. 62, 5276–5297 (2019).
Li, L., Jakowski, J., Do, C. & Hong, K. Deuteration and polymers: rich history with great potential. Macromolecules 54, 3555–3584 (2021).
Schoenheimer, R. & Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism. Science 82, 156–157 (1935).
De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 4, eaat7314 (2018).
Zhang, L. et al. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 3, 402–413 (2019).
Miyashita, M., Sasaki, M., Hattori, I., Sakai, M. & Tanino, K. Total synthesis of norzoanthamine. Science 305, 495–499 (2004).
Gómez-Gallego, M. & Sierra, M. A. Kinetic isotope effects in the study of organometallic reaction mechanisms. Chem. Rev. 111, 4857–4963 (2011).
Zuo, Z. et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science 345, 437–440 (2014).
Patra, T. & Maiti, D. Decarboxylation as the key step in C–C bond-forming reactions. Chem. Eur. J. 23, 7382–7401 (2017).
Sajiki, H., Aoki, F., Esaki, H., Maegawa, T. & Hirota, K. Efficient C–H/C–D exchange reaction on the alkyl side chain of aromatic compounds using heterogeneous Pd/C in D2O. Org. Lett. 6, 1485–1487 (2004).
Esaki, H. et al. Efficient H/D exchange reactions of alkyl-substituted benzene derivatives by means of the Pd/C–H2–D2O system. Chem. Eur. J. 13, 4052–4063 (2007).
Yamada, T. et al. Mild and direct multiple deuterium-labeling of saturated fatty acids. Adv. Synth. Catal. 358, 3277–3282 (2016).
Naruto, M., Agrawal, S., Toda, K. & Saito, S. Catalytic transformation of functionalized carboxylic acids using multifunctional rhenium complexes. Sci Rep. 7, 3425 (2017).
Uttry, A., Mal, S. & van Gemmeren, M. Late-stage β-C(sp3)–H deuteration of carboxylic acids. J. Am. Chem. Soc. 143, 10895–10901 (2021).
Atkinson, J. G., Csakvary, J. J., Herbert, G. T. & Stuart, R. S. Exchange reactions of carboxylic acid salts. Facile preparation of α-deuteriocarboxylic acids. J. Am. Chem. Soc. 90, 498–499 (1968).
Yu, K. et al. Lithium enolates in the enantioselective construction of tetrasubstituted carbon centers with chiral lithium amides as noncovalent stereodirecting auxiliaries. J. Am. Chem. Soc. 139, 527–533 (2017).
Evans, D. A., Nelson, J. V., Vogel, E. & Taber, T. R. Stereoselective condensations via boron enolates. J. Am. Chem. Soc. 103, 3099–3111 (1981).
Morita, Y., Yamamoto, T., Nagai, H., Shimizu, Y. & Kanai, M. Chemoselective boron-catalyzed nucleophilic activation of carboxylic acids for Mannich-type reactions. J. Am. Chem. Soc. 137, 7075–7078 (2015).
Kotani, S., Yoshiwara, Y., Ogasawara, M., Sugiura, M. & Nakajima, M. Catalytic enantioselective aldol reactions of unprotected carboxylic acids under phosphine oxide catalysis. Angew. Chem. Int. Ed. 57, 15877–15881 (2018).
Tanaka, T., Yazaki, R. & Ohshima, T. Chemoselective catalytic α-oxidation of carboxylic acids: iron/alkali metal cooperative redox active catalysis. J. Am. Chem. Soc. 142, 4517–4524 (2020).
Perkin, W. H. XXIII.–On the hydride of aceto-salicyl. J. Chem. Soc. 21, 181–192 (1868).
Dmitrevskaya, L. I. et al. New method for α-deuteration of carboxylic acids. Zh. Org. Khim. 19, 2278–2281 (1983).
Morrill, L. C. & Smith, A. D. Organocatalytic Lewis base functionalisation of carboxylic acids, esters and anhydrides via C1-ammonium or azolium enolates. Chem. Soc. Rev. 43, 6214–6226 (2014).
Steglich, W. & Höfle, G. N,N‐Dimethyl‐4‐pyridinamine, a very effective acylation catalyst. Angew. Chem. Int. Ed. 8, 981–981 (1969).
Chang, Y. et al. Catalytic deuterium incorporation within metabolically stable β-amino C–H bonds of drug molecules. J. Am. Chem. Soc. 141, 14570–14575 (2019).
Berthelette, C. & Scheigetz, J. Base-catalyzed deuterium and tritium labelling of 1-biphenyl-4-ylpropane-1,2-dione and deuteration of aryl methyl ketones. J. Labelled Comp. Radiopharm. 47, 891–894 (2004).
Zhan, M., Zhang, T., Huang, H., Xie, Y. & Chen, Y. A simple method for α-Position deuterated carbonyl compounds with pyrrolidine as catalyst. J. Labelled Comp. Radiopharm. 57, 533–539 (2014).
Chang, Y., Myers, T. & Wasa, M. B(C6F5)3-catalyzed α-deuteration of bioactive carbonyl compounds with D2O. Adv. Synth. Catal. 362, 360–364 (2020).
Yang, C., Xue, X. S., Jin, J. L., Li, X. & Cheng, J. P. Theoretical study on the acidities of chiral phosphoric acids in dimethyl sulfoxide: hints for organocatalysis. J. Org. Chem. 78, 7076–7085 (2013).
Zhang, Z., Zheng, Y., Xiao-Song, X. & Pengju, J. A systematic theoretical study on the acidities for cations of ionic liquids in dimethyl sulfoxide. J. Phys. Chem. A 122, 5750–5755 (2018).
Huihui, K. M. M. et al. Decarboxylative cross-electrophile coupling of N-hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc. 138, 5016–5019 (2016).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Fu, M. C., Shang, R., Zhao, B., Wang, B. & Fu, Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science 363, 1429–1434 (2019).
Patra, T., Mukherjee, S., Ma, J., Strieth‐Kalthoff, F. & Glorius, F. Visible‐light‐photosensitized aryl and alkyl decarboxylative functionalization reactions. Angew. Chem. Int. Ed. 131, 10624–10630 (2019).
Li, N. et al. A highly selective decarboxylative deuteration of carboxylic acids. Chem. Sci. 12, 5505–5510 (2021).
Asada, M. et al. 3-(2-Aminocarbonylphenyl)propanoic acid analogs as potent and selective EP3 receptor antagonists. Part 1: discovery and exploration of the carboxyamide side chain. Bioorg. Med. Chem. 18, 80–90 (2010).
Kawajiri, T. et al. Chemoselective nucleophilic functionalizations of aromatic aldehydes and acetals via pyridinium salt intermediates. J. Org. Chem. 84, 3853–3870 (2019).
Farizyan, M., Mondal, A., Mal, S., Deufel, F. & Van Gemmeren, M. Palladium-catalyzed nondirected late-stage C–H deuteration of arenes. J. Am. Chem. Soc. 143, 16370–16376 (2021).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Takahashi, M., Oshima, K. & Matsubara, S. Ruthenium catalyzed deuterium labelling of α-carbon in primary alcohol and primary/secondary amine in D2O. Chem. Lett. 34, 192–193 (2005).
Neubert, L. et al. Ruthenium-catalyzed selective α,β-deuteration of bioactive amines. J. Am. Chem. Soc. 134, 12239–12244 (2012).
Loh, Y. Y. et al. Photoredoxcatalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Acknowledgements
This work was financially supported by MEXT KAKENHI grant numbers JP21A204, JP21H05207 and JP21H05208 for The Grant-in-Aid for Transformative Research Areas (A) Digitalization-driven Transformative Organic Synthesis (Digi-TOS) from MEXT, JSPS KAKENHI grant no. JP15H05846 in Middle Molecular Strategy, no. JP18H04263 in Precisely Designed Catalysts with Customized Scaffolding, a Grant-in-Aid for Scientific Research (B) (no. JP17H03972 and no. JP21H02607), a Grant-in-Aid for Challenging Research (Exploratory) (no. JP19K22501) from JSPS, Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS) (no. JP20am0101091 and no. JP22ama121031) and no. JP21ak0101167 from AMED. T.T. thanks the JSPS for a predoctoral fellowship. R.Y. thanks the Qdai-jump Research Program, the Ube Industries Foundation, the Amano Institute of Technology, the ENEOS Tonengeneral Research/Development Encouragement & Scholarship Foundation, the Takeda Science Foundation, the Noguchi Institute and the Astellas Foundation for Research on Metabolic Disorders for financial support. We are grateful to H. Morimoto at Kyushu University for fruitful discussions about the density functional theory calculations.
Author information
Authors and Affiliations
Contributions
T.T., R.Y. and T.O. conceived the work, analysed the data and discussed the results, and all of the authors co-wrote the manuscript. T.T. designed and carried out the experiments with Y.K. and Y.H. The human microsomal metabolic assay was performed by A.T.
Corresponding authors
Ethics declarations
Competing interests
A provisional patent application for part of this work has been filed (Kyushu University, application number 2021-147127).
Peer review
Peer review information
Nature Synthesis thanks Jin Xie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, T., Koga, Y., Honda, Y. et al. Ternary catalytic α-deuteration of carboxylic acids. Nat. Synth 1, 824–830 (2022). https://doi.org/10.1038/s44160-022-00139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00139-9