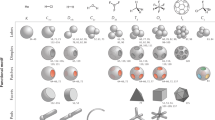
Abstract
Over the past 40 years, scientists have developed routes to synthesize colloidal nanocrystals of different compositions and with tunable size and shape. These features dictate the properties of these nanomaterials and, thus, their control aids the discovery of different physical chemical phenomena, many of which have contributed to technological advances; for example, the use of semiconductor nanocrystals as active components in displays with excellent colour purity. Yet, the synthesis of colloidal nanocrystals still proceeds by trial and error. The search for the reaction conditions to obtain nanocrystals with the desired compositions, sizes and shapes is time consuming and can fail to deliver the target product. In this Perspective, we discuss the importance of identifying reaction intermediates during the formation of colloidal nanocrystals for the development of a retrosynthetic approach to these nanomaterials. We select molecular complexes and clusters, coordination polymers and mesophases, and inorganic nanoparticles as some of the most common intermediates. The discovered pathways pinpoint the steps that enable a more predictive synthesis of colloidal nanocrystals. This Perspective encourages more mechanistic studies to eventually implement the concept of retrosynthesis for these nanomaterials.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Efros, A. L. & Brus, L. E. Nanocrystal quantum dots: from discovery to modern development. ACS Nano 15, 6192–6210 (2021).
Kovalenko, M. V. et al. Prospects of nanoscience with nanocrystals. ACS Nano 9, 1012–1057 (2015).
Eustis, S. & El-Sayed, M. A. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 35, 209–217 (2006).
Saha, K., Agasti, S. S., Kim, C., Li, X. & Rotello, V. M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112, 2739–2779 (2021).
Guntern, Y. T. et al. Colloidal nanocrystals as electrocatalysts with tunable activity and selectivity. ACS Catal. 11, 1248–1295 (2021).
Cargnello, M. Colloidal nanocrystals as building blocks for well-defined heterogeneous catalysts. Chem. Mater. 31, 576–596 (2019).
Witte, P. T. et al. BASF NanoSelect technology: innovative supported Pd- and Pt-based catalysts for selective hydrogenation reactions. Top. Catal. 55, 505–511 (2012).
Chen, X. & Mao, S. S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 107, 2891–2959 (2007).
Wu, L., Mendoza-Garcia, A., Li, Q. & Sun, S. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 116, 10473–11051 (2016).
Buonsanti, R., Loiudice, A. & Mantella, V. Colloidal nanocrystals as precursors and intermediates in solid state reactions for multinary oxide nanomaterials. Acc. Chem. Res. 54, 754–764 (2021).
Heuer-Jungemann, A. et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 119, 4819–4880 (2019).
LaMer, V. K. & Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 72, 4847–4854 (1950).
Kwon, S. G. & Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 7, 2685–2702 (2011).
Lee, J., Yang, J., Kwon, S. G. & Hyeon, T. Nonclassical nucleation and growth of inorganic nanoparticles. Nat. Rev. Mater. 1, 16034 (2016).
Nicolalaou, K. C. Organic synthesis: the art and science of replicating the molecules of living nature and creating others like them in the laboratory. Proc. R. Soc A. 470, 20130690 (2014).
Koziej, D. Revealing complexity of nanoparticle synthesis in solution by in situ hard X-ray spectroscopy - today and beyond. Chem. Mater. 28, 2478–2490 (2016).
Wu, S., Li, M. & Sun, Y. In situ synchrotron X‐ray characterization shining light on the nucleation and growth kinetics of colloidal nanoparticles. Angew. Chem. Int. Ed. 131, 9083–9091 (2019).
Peng, X. G. et al. Shape control of CdSe nanocrystals. Nature 404, 59–61 (2000).
Peng, Z. A. & Peng, X. Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J. Am. Chem. Soc. 124, 3343–3353 (2002).
Riedinger, A. et al. An intrinsic growth instability in isotropic materials leads to quasi-two-dimensional nanoplatelets. Nat. Mater. 16, 743–748 (2017).
Cunningham, P. D., Coropceanu, I., Mulloy, K., Cho, W. & Talapin, D. V. Quantized reaction pathways for solution synthesis of colloidal ZnSe nanostructures: a connection between clusters, nanowires, and two-dimensional nanoplatelets. ACS Nano 14, 3847–3857 (2020).
Ning, J., Liu, J., Levi-Kalisman, Y., Frenkel, A. I. & Banin, U. Controlling anisotropic growth of colloidal ZnSe nanostructures. J. Am. Chem. Soc. 140, 14627–14637 (2018).
Gary, D. C., Terban, M. W., Billinge, S. J. L. & Cossairt, B. M. Two-step nucleation and growth of InP quantum dots via magic-sized cluster intermediates. Chem. Mater. 27, 1432–1441 (2015).
Gary, D. C. et al. Single-crystal and electronic structure of a 1.3 nm indium phosphide nanocluster. J. Am. Chem. Soc. 138, 1510–1513 (2016).
Friedfeld, M. R., Stein, J. L. & Cossairt, B. M. Main-group-semiconductor cluster molecules as synthetic intermediates to nanostructures. Inorg. Chem. 56, 8689–8697 (2017).
Mule, A. S. et al. Unraveling the growth mechanism of magic-sized semiconductor nanocrystals. J. Am. Chem. Soc. 143, 2037–2048 (2021).
Harris, D. K. & Bawendi, M. G. Improved precursor chemistry for the synthesis of III–V quantum dots. J. Am. Chem. Soc. 134, 20211–20213 (2012).
Gary, D. C., Glassy, B. A. & Cossairt, B. M. Investigation of indium phosphide quantum dot nucleation and growth utilizing triarylsilylphosphine precursors. Chem. Mater. 26, 1734–1744 (2014).
Xie, R., Battaglia, D. & Peng, X. Colloidal InP nanocrystals as efficient emitters covering blue to near-infrared. J. Am. Chem. Soc. 129, 15432–15433 (2007).
Chang, H. et al. Molecular-level understanding of continuous growth from iron–oxo clusters to iron oxide nanoparticles. J. Am. Chem. Soc. 141, 7037–7045 (2019).
Kwon, S. G. et al. Kinetics of monodisperse iron oxide nanocrystal formation by ‘heating-up’ process. J. Am. Chem. Soc. 129, 12571–12584 (2007).
Feld, A. et al. Chemistry of shape-controlled iron oxide nanocrystal formation. ACS Nano 13, 152–162 (2019).
Strach, M. et al. Insights into reaction intermediates to predict synthetic pathways for shape-controlled metal nanocrystals. J. Am. Chem. Soc. 141, 16312–16322 (2019).
Mantella, V., Castilla-Amorós, L. & Buonsanti, R. Shaping non-noble metal nanocrystals: via colloidal chemistry. Chem. Sci. 11, 11394–11403 (2020).
Xia, Y., Xiong, Y., Lim, B. & Skrabalak, S. E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics. Angew. Chem. Int. Ed. 48, 60–103 (2009).
Porter, R. S., Barrall, E. M. II & Johnson, J. F. Order and flow of liquid crystals. XVI. Thermodynamic order in mesophases. Acc. Chem. Res. 2, 53–58 (1969).
Nevers, D. R. et al. Mesophase formation stabilizes high-purity magic-sized clusters. J. Am. Chem. Soc. 140, 3652–3662 (2018).
Mantella, V. et al. Polymer lamellae as reaction intermediates in the formation of copper nanospheres as evidenced by in situ X-ray studies. Angew. Chem. Int. Ed. 59, 11627–11633 (2020).
Gromova, M. et al. Growth mechanism and surface state of CuInS2 nanocrystals synthesized with dodecanethiol. J. Am. Chem. Soc. 139, 15748–15759 (2017).
Son, J. S. et al. Large-scale soft colloidal template synthesis of 1.4 nm thick CdSe nanosheets. Angew. Chem. Int. Ed. 48, 6861–6864 (2009).
Yang, J., Son, J. S., Yu, J. H., Joo, J. & Hyeon, T. Advances in the colloidal synthesis of two-dimensional semiconductor nanoribbons. Chem. Mater. 25, 1190–1198 (2013).
Liu, Y. H., Wang, F., Wang, Y., Gibbons, P. C. & Buhro, W. E. Lamellar assembly of cadmium selenide nanoclusters into quantum belts. J. Am. Chem. Soc. 133, 17005–17013 (2011).
Loh, N. D. et al. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 9, 77–82 (2017).
Yang, J. et al. Amorphous-phase-mediated crystallization of Ni nanocrystals revealed by high-resolution liquid-phase electron microscopy. J. Am. Chem. Soc. 141, 763–768 (2019).
Son, J. S. et al. Dimension-controlled synthesis of CdS nanocrystals: from 0D quantum dots to 2D nanoplates. Small 8, 2394–2402 (2012).
Yu, J. H. et al. Giant Zeeman splitting in nucleation-controlled doped CdSe:Mn2+ quantum nanoribbons. Nat. Mater. 9, 47–53 (2010).
de la Mata, M. et al. A review of MBE grown 0D, 1D and 2D quantum structures in a nanowire. J. Mater. Chem. C 1, 4300–4312 (2013).
Wang, F., Richards, V. N., Shields, S. P. & Buhro, W. E. Kinetics and mechanisms of aggregative nanocrystal growth. Chem. Mater. 26, 5–21 (2014).
Salzmann, B. B. V., van der Sluijs, M. M., Soligno, G. & Vanmaekelbergh, D. Oriented attachment: from natural crystal growth to a materials engineering tool. Acc. Chem. Res. 54, 787–797 (2021).
Li, D. et al. Direction-specific interactions control crystal growth by oriented attachment. Science 336, 1014–1018 (2012).
Penn, R. L. & Banfield, J. F. Imperfect oriented attachment: dislocation generation in defect-free nanocrystals. Science 281, 969–971 (1998).
Pacholski, C., Kornowski, A. & Weller, H. Self-assembly of ZnO: from nanodots to nanorods. Angew. Chem. Int. Ed. 41, 1188–1191 (2002).
Schliehe, C. et al. Ultrathin PbS sheets by two-dimensional oriented attachment. Science 329, 550–553 (2010).
Liao, H. G., Cui, L., Whitelam, S. & Zheng, H. Real-time imaging of Pt3Fe nanorod growth in solution. Science 336, 1011–1014 (2012).
Whitham, K. et al. Charge transport and localization in atomically coherent quantum dot solids. Nat. Mater. 15, 557–563 (2016).
Delerue, C. Order and progress. Nat. Mater. 15, 498–499 (2016).
Baumgardner, W. J., Withhaman, K. & Hanrath, T. Confined-but-connected quantum dot solids via controlled ligand displacement. Nano Lett. 13, 3225–3231 (2013).
De Trizio, L. & Manna, L. Forging colloidal nanostructures via cation exchange reactions. Chem. Rev. 116, 10852–10887 (2016).
Rivest, J. B. & Jain, P. K. Cation exchange on the nanoscale: an emerging technique for new material synthesis, device fabrication, and chemical sensing. Chem. Soc. Rev. 42, 89–96 (2013).
Fenton, J. L., Steimle, B. C. & Schaak, R. E. Tunable intraparticle frameworks for creating complex heterostructured nanoparticle libraries. Science 560, 513–517 (2018).
Steimle, B. C., Fenton, J. L. & Schaak, R. E. Rational construction of a scalable heterostructured nanorod megalibrary. Science 367, 418–424 (2020).
Mantella, V. et al. Synthesis and size-dependent optical properties of intermediate band gap Cu3VS4 nanocrystals. Chem. Mater. 31, 532–540 (2019).
Mantella, V., Varandili, S. B., Pankhurst, J. R. & Buonsanti, R. Colloidal synthesis of Cu−M−S (M = V, Cr, Mn) nanocrystals by tuning the copper precursor reactivity. Chem. Mater. 32, 9780–9786 (2020).
Muthuswamy, E. & Brock, S. L. Solid-state phase transformations in solution: templated conversion of nanoscale nickel phosphides. Chem. Commun. 47, 12334–12336 (2011).
Shevchenko, E. V. et al. Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: the role of nucleation rate in size control of CoPt3 nanocrystals. J. Am. Chem. Soc. 125, 9090–9101 (2003).
Schaak, R. E. et al. Metallurgy in a beaker: nanoparticle toolkit for the rapid low-temperature solution synthesis of functional multimetallic solid-state materials. J. Am. Chem. Soc. 127, 3506–3515 (2005).
Gadiyar, C. et al. Nanocrystals as precursors in solid-state reactions for size- and shape-controlled polyelemental nanomaterials. J. Am. Chem. Soc. 142, 15931–15940 (2020).
Lee, J. M., Kraynak, L. A. & Prieto, A. L. A directed route to colloidal nanoparticle synthesis of the copper selenophosphate Cu3PSe4. Angew. Int. Chem. Ed. 132, 3062–3066 (2020).
Krishnadasan, S., Brown, R. J. C., deMello, A. J. & DeMello, J. C. Intelligent routes to the controlled synthesis of nanoparticles. Lab Chip 7, 1434–1441 (2007).
Rakita, Y. et al. Active reaction control of Cu redox state based on real-time feedback from in situ synchrotron measurements. J. Am. Chem. Soc. 142, 18758–18762 (2020).
Schwaller, P. et al. Predicting retrosynthetic pathways using transformer-based models and a hyper-graph exploration strategy. Chem. Sci. 11, 3316–3325 (2020).
Aykol, M., Montoya, J. H. & Hummelshøj, J. Rational solid-state synthesis routes for inorganic materials. J. Am. Chem. Soc. 143, 9244–9259 (2021).
Kovnir, K. Predictive synthesis. Chem. Mater. 33, 4835–4841 (2021).
Acknowledgements
The authors acknowledge the great support of the École Politechnique Fédérale de Lausanne over the past six years.
Author information
Authors and Affiliations
Contributions
A.L. and R.B. discussed the content and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Brian Korgel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loiudice, A., Buonsanti, R. Reaction intermediates in the synthesis of colloidal nanocrystals. Nat. Synth 1, 344–351 (2022). https://doi.org/10.1038/s44160-022-00056-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00056-x
This article is cited by
-
Assignment of individual structures from intermetalloid nickel gallium cluster ensembles
Communications Chemistry (2024)
-
Effect of ligand dispersant on superparamagnetic heating capacity and cytotoxicity properties of MnFe2O4 nanoparticles
Journal of Materials Science: Materials in Electronics (2024)
-
Retrosynthetic design of core–shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles
Nature Synthesis (2023)
-
Photothermally heated colloidal synthesis of nanoparticles driven by silica-encapsulated plasmonic heat sources
Nature Communications (2023)