Abstract
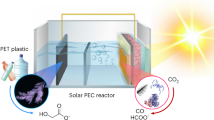
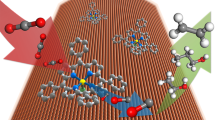
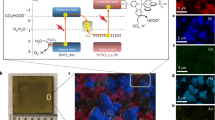
The reduction of CO2 to renewable fuels must be coupled to a sustainable oxidation process to devise a viable device that produces solar fuels. In photoelectrochemical cells, water oxidation to O2 is the predominant oxidation reaction and typically requires a pair of light absorbers or an applied bias voltage when coupled to CO2 reduction. Here, we report a bias-free photoelectrochemical device for simultaneous CO2 reduction to formate and alcohol oxidation to aldehyde in aqueous conditions. The photoanode is constructed by co-immobilization of a diketopyrrolopyrrole-based chromophore and a nitroxyl-based alcohol oxidation catalyst on a mesoporous TiO2 scaffold, which provides a precious-metal-free dye-sensitized photoanode. The photoanode is wired to a biohybrid cathode that consists of the CO2 reduction enzyme formate dehydrogenase integrated into a mesoporous indium tin oxide electrode. The bias-free cell delivers sustained photocurrents of up to 30 µA cm−2 under visible-light irradiation, which results in simultaneous aldehyde and formate production. Our results show that in the absence of an external bias, single light absorber photoelectrochemical cells can be used for parallel fuel production and chemical synthesis from CO2 and alcohol substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of the study are available in the paper and its Supplementary Information. Other source data supporting the findings of this study are available from the Cambridge data repository (https://doi.org/10.17863/CAM.76484). Source data are provided with this paper.
References
Appel, A. M. et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 113, 6621–6658 (2013).
Artz, J. et al. Sustainable conversion of carbon dioxide: an integrated review of catalysis and life cycle assessment. Chem. Rev. 118, 434–504 (2018).
You, B., Liu, X., Jiang, N. & Sun, Y. A general strategy for decoupled hydrogen production from water splitting by integrating oxidative biomass valorization. J. Am. Chem. Soc. 138, 13639–13646 (2016).
Shaner, M. R., Atwater, H. A., Lewis, N. S. & McFarland, E. W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 9, 2354–2371 (2016).
Na, J. et al. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 10, 5193 (2019).
Reid, L. M., Li, T., Cao, Y. & Berlinguette, C. P. Organic chemistry at anodes and photoanodes. Sustain. Energy Fuels 2, 1905–1927 (2018).
Uekert, T., Pichler, C. M., Schubert, T. & Reisner, E. Solar-driven reforming of solid waste for a sustainable future. Nat. Sustain. 4, 383–391 (2021).
Rosatella, A. A., Simeonov, S. P., Frade, R. F. M. & Afonso, C. A. M. 5-Hydroxymethylfurfural (HMF) as a building block platform: biological properties, synthesis and synthetic applications. Green Chem. 13, 754–793 (2011).
Wu, Y.-C., Song, R.-J. & Li, J.-H. Recent advances in photoelectrochemical cells (PECs) for organic synthesis. Org. Chem. Front. 7, 1895–1902 (2020).
Cha, H. G. & Choi, K.-S. Combined biomass valorization and hydrogen production in a photoelectrochemical cell. Nat. Chem. 7, 328–333 (2015).
Li, C., Zhao, X., Wang, A., Huber, G. W. & Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 115, 11559–11624 (2015).
Wang, Y. et al. Simultaneous electrosynthesis of syngas and an aldehyde from CO2 and an alcohol by molecular electrocatalysis. ACS Appl. Energy Mater. 2, 97–101 (2019).
Li, T., Cao, Y., He, J. & Berlinguette, C. P. Electrolytic CO2 reduction in tandem with oxidative organic chemistry. ACS Cent. Sci. 3, 778–783 (2017).
Bajada, M. A. et al. A precious‐metal‐free hybrid electrolyzer for alcohol oxidation coupled to CO2‐to‐syngas conversion. Angew. Chem. Int. Ed. 59, 15633–15641 (2020).
Wang, L., Zhang, X., Yang, L., Wang, C. & Wang, H. Photocatalytic reduction of CO2 coupled with selective alcohol oxidation under ambient conditions. Catal. Sci. Technol. 5, 4800–4805 (2015).
Chen, Y. et al. Coupling photocatalytic CO2 reduction with benzyl alcohol oxidation to produce benzyl acetate over Cu2O/Cu. Catal. Sci. Technol. 8, 2218–2223 (2018).
Spitler, M. T. et al. Practical challenges in the development of photoelectrochemical solar fuels production. Sustain. Energy Fuels 4, 985–995 (2020).
Harris, A. W., Yehezkeli, O., Hafenstine, G. R., Goodwin, A. P. & Cha, J. N. Light-driven catalytic upgrading of butanol in a biohybrid photoelectrochemical system. ACS Sustain. Chem. Eng. 5, 8199–8204 (2017).
Li, T. et al. Photoelectrochemical oxidation of organic substrates in organic media. Nat. Commun. 8, 390 (2017).
Wang, D. et al. Lignin-fueled photoelectrochemical platform for light-driven redox biotransformation. Green Chem. 22, 5151–5160 (2020).
Seabold, J. A. & Choi, K.-S. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem. Mater. 23, 1105–1112 (2011).
Bella, F., Gerbaldi, C., Barolo, C. & Grätzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 44, 3431–3473 (2015).
Grätzel, M. Photoelectrochemical cells. Nature 414, 338–344 (2001).
Kavan, L., Tétreault, N., Moehl, T. & Grätzel, M. Electrochemical characterization of TiO2 blocking layers for dye-sensitized solar cells. J. Phys. Chem. C 118, 16408–16418 (2014).
Song, W. et al. Visible light driven benzyl alcohol dehydrogenation in a dye-sensitized photoelectrosynthesis cell. J. Am. Chem. Soc. 136, 9773–9779 (2014).
Pho, T. V. et al. Efficient light-driven oxidation of alcohols using an organic chromophore–catalyst assembly anchored to TiO2. ACS Appl. Mater. Interfaces 8, 9125–9133 (2016).
Badgurjar, D. et al. Electron-withdrawing boron dipyrromethene dyes as visible light absorber/sensitizers on semiconductor oxide surfaces. ACS Appl. Mater. Interfaces 12, 7768–7776 (2020).
Creissen, C. E., Warnan, J. & Reisner, E. Solar H2 generation in water with a CuCrO2 photocathode modified with an organic dye and molecular Ni catalyst. Chem. Sci. 9, 1439–1447 (2018).
Antón-García, D., Warnan, J. & Reisner, E. A diketopyrrolopyrrole dye-based dyad on a porous TiO2 photoanode for solar-driven water oxidation. Chem. Sci. 11, 12769–12776 (2020).
Brennaman, M. K. et al. Finding the way to solar fuels with dye-sensitized photoelectrosynthesis cells. J. Am. Chem. Soc. 138, 13085–13102 (2016).
Sokol, K. P. et al. Bias-free photoelectrochemical water splitting with photosystem II on a dye-sensitized photoanode wired to hydrogenase. Nat. Energy 3, 944–951 (2018).
Miller, M. et al. Interfacing formate dehydrogenase with metal oxides for the reversible electrocatalysis and solar-driven reduction of carbon dioxide. Angew. Chem. Int. Ed. 58, 4601–4605 (2019).
Oliveira, A. R. et al. Toward the mechanistic understanding of enzymatic CO2 reduction. ACS Catal. 10, 3844–3856 (2020).
Warnan, J. et al. A compact diketopyrrolopyrrole dye as efficient sensitizer in titanium dioxide dye-sensitized solar cells. J. Photochem. Photobiol. A 226, 9–15 (2011).
Leung, J. J. et al. Solar-driven reduction of aqueous CO2 with a cobalt bis(terpyridine)-based photocathode. Nat. Catal. 2, 354–365 (2019).
Hoertz, P. G., Chen, Z., Kent, C. A. & Meyer, T. J. Application of high surface area tin-doped indium oxide nanoparticle films as transparent conducting electrodes. Inorg. Chem. 49, 8179–8181 (2010).
Zigler, D. F. et al. Disentangling the physical processes responsible for the kinetic complexity in interfacial electron transfer of excited Ru(II) polypyridyl dyes on TiO2. J. Am. Chem. Soc. 138, 4426–4438 (2016).
Xu, P., McCool, N. S. & Mallouk, T. E. Water splitting dye-sensitized solar cells. Nano Today 14, 42–58 (2017).
Willkomm, J. et al. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 45, 9–23 (2016).
Leung, J. J. et al. Photoelectrocatalytic H2 evolution in water with molecular catalysts immobilised on p-Si via a stabilising mesoporous TiO2 interlayer. Chem. Sci. 8, 5172–5180 (2017).
Schreier, M. et al. Covalent immobilization of a molecular catalyst on Cu2O photocathodes for CO2 reduction. J. Am. Chem. Soc. 138, 1938–1946 (2016).
Kay, A. & Grätzel, M. Artificial photosynthesis. 1. Photosensitization of titania solar cells with chlorophyll derivatives and related natural porphyrins. J. Phys. Chem. 97, 6272–6277 (1993).
Manthou, V. S., Pefkianakis, E. K., Falaras, P. & Vougioukalakis, G. C. Co-adsorbents: a key component in efficient and robust dye-sensitized solar cells. ChemSusChem 8, 588–599 (2015).
Bruggeman, D. F., Bakker, T. M. A., Mathew, S. & Reek, J. N. H. Redox‐mediated alcohol oxidation coupled to hydrogen gas formation in a dye‐sensitized photosynthesis cell. Chem. Eur. J. 27, 218–221 (2021).
Bullock, R. M., Das, A. K. & Appel, A. M. Surface immobilization of molecular electrocatalysts for energy conversion. Chem. Eur. J. 23, 7626–7641 (2017).
Wang, D. et al. Stabilized photoanodes for water oxidation by integration of organic dyes, water oxidation catalysts, and electron-transfer mediators. Proc. Natl Acad. Sci. USA 115, 8523–8528 (2018).
Suryani, O. et al. A near-infrared organic photosensitizer for use in dye-sensitized photoelectrochemical water splitting. Chem. Commun. 53, 6784–6787 (2017).
Yamamoto, M. et al. Visible light-driven water oxidation with a subporphyrin sensitizer and a water oxidation catalyst. Chem. Commun. 52, 13702–13705 (2016).
Chadderdon, X. H., Chadderdon, D. J., Pfennig, T., Shanks, B. H. & Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 21, 6210–6219 (2019).
Kashparova, V. P. et al. Selective synthesis of 2,5-diformylfuran by sustainable 4-acetamido-TEMPO/halogen-mediated electrooxidation of 5-hydroxymethylfurfural. Chem. Asian J. 11, 2578–2585 (2016).
Rafiee, M., Konz, Z. M., Graaf, M. D., Koolman, H. F. & Stahl, S. S. Electrochemical oxidation of alcohols and aldehydes to carboxylic acids catalyzed by 4-acetamido-TEMPO: an alternative to ‘Anelli’ and ‘Pinnick’ oxidations. ACS Catal. 8, 6738–6744 (2018).
Fang, X. et al. Structure–activity relationships of hierarchical three-dimensional electrodes with photosystem II for semiartificial photosynthesis. Nano Lett. 19, 1844–1850 (2019).
Green, A. N. M., Palomares, E., Haque, S. A., Kroon, J. M. & Durrant, J. R. Charge transport versus recombination in dye-sensitized solar cells employing nanocrystalline TiO2 and SnO2 films. J. Phys. Chem. B 109, 12525–12533 (2005).
Sokol, K. P. et al. Photoreduction of CO2 with a formate dehydrogenase driven by photosystem II using a semi-artificial Z-scheme architecture. J. Am. Chem. Soc. 140, 16418–16422 (2018).
Windle, C. D., Massin, J., Chavarot-Kerlidou, M. & Artero, V. A protocol for quantifying hydrogen evolution by dye-sensitized molecular photocathodes and its implementation for evaluating a new covalent architecture based on an optimized dye–catalyst dyad. Dalton Trans. 47, 10509–10516 (2018).
Coridan, R. H. et al. Methods for comparing the performance of energy-conversion systems for use in solar fuels and solar electricity generation. Energy Environ. Sci. 8, 2886–2901 (2015).
Liu, C., Colón, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Wang, Q. et al. Molecularly engineered photocatalyst sheet for scalable solar formate production from carbon dioxide and water. Nat. Energy 5, 703–710 (2020).
Kim, J. H., Hansora, D., Sharma, P., Jang, J.-W. & Lee, J.-S. Toward practical solar hydrogen production—an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Chen, H. et al. Fundamentals, applications, and future directions of bioelectrocatalysis. Chem. Rev. 120, 12903–12993 (2020).
Plumeré, N. et al. A redox hydrogel protects hydrogenase from high-potential deactivation and oxygen damage. Nat. Chem. 6, 822–827 (2014).
Dalle, K. E. et al. Electro- and solar-driven fuel synthesis with first row transition metal complexes. Chem. Rev. 119, 2752–2875 (2019).
Kornienko, N., Zhang, J. Z., Sakimoto, K. K., Yang, P. & Reisner, E. Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis. Nat. Nanotechnol. 13, 890–899 (2018).
Son, E. J. et al. Sunlight-assisted, biocatalytic formate synthesis from CO2 and water using silicon-based photoelectrochemical cells. Chem. Commun. 52, 9723–9726 (2016).
Kuk, S. K. et al. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade. Angew. Chem. Int. Ed. 56, 3827–3832 (2017).
Nam, D. H. et al. Enzymatic photosynthesis of formate from carbon dioxide coupled with highly efficient photoelectrochemical regeneration of nicotinamide cofactors. Green Chem. 18, 5989–5993 (2016).
Kuk, S. K. et al. CO2‐reductive, copper oxide‐based photobiocathode for Z‐scheme semi‐artificial leaf structure. ChemSusChem 13, 2940–2944 (2020).
Lee, S. Y., Lim, S. Y., Seo, D., Lee, J.-Y. & Chung, T. D. Light-driven highly selective conversion of CO2 to formate by electrosynthesized enzyme/cofactor thin film electrode. Adv. Energy Mater. 6, 1502207 (2016).
Ishibashi, T., Higashi, M., Ikeda, S. & Amao, Y. Photoelectrochemical CO2 reduction to formate with the sacrificial reagent free system of semiconductor photocatalysts and formate dehydrogenase. ChemCatChem 11, 6227–6235 (2019).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian Inc., 2013).
Farré, Y. et al. Second generation of diketopyrrolopyrrole dyes for NiO-based dye-sensitized solar cells. J. Phys. Chem. C 120, 7923–7940 (2016).
Hehre, W. J., Stewart, R. F. & Pople, J. A. Self-consistent molecular-orbital methods. I. Use of gaussian expansions of Slater-type atomic orbitals. J. Chem. Phys. 51, 2657–2664 (1969).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Tomasi, J., Mennucci, B. & Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 105, 2999–3093 (2005).
Yanai, T., Tew, D. P. & Handy, N. C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004).
Sawyer, D. T., Sobkowiak, A. & Roberts, J. L. Electrochemistry for Chemists (Wiley-Interscience, 1995).
Acknowledgements
We acknowledge support from an EPSRC PhD DTA studentship (EP/M508007/1, D.A.-G.), the Christian Doppler Research Association (Austrian Federal Ministry for Digital and Economic Affairs and the National Foundation for Research, Technology and Development) and the OMV Group (M.A.B., J.W. and E.R.), an ERC Consolidator Grant ‘MatEnSAP’ (682833; D.A.-G., E.E.M. and E.R.), the Endeavour Scholarship Scheme (M.A.B.), the German National Academy of Sciences Leopoldina for a postdoctoral fellowship (LPDS 2018-04, A.E.), Fundação para a Ciência e Tecnologia (Portugal) for fellowship SFRH/BD/116515/2016 (A.R.O.), grant PTDC/BII-BBF/2050/2020 (I.A.C.P.) and R&D unit MOSTMICRO-ITQB (UIDB/04612/2020 and UIDP/04612/2020). J.W. gratefully acknowledges support from R. Fischer and the Deutsche Forschungsgemeinschaft (grant no. FI 502/43-1). We thank Q. Wang, N. Kornienko, C. Pichler and A. Wagner for helpful discussions. We also thank N. Heidary and K. Ly for their help in preparing the artwork. We appreciate suggestions and comments on the manuscript from T. Li and Q. Wang.
Author information
Authors and Affiliations
Contributions
D.A.-G., J.W. and E.R. designed the project. D.A.-G. synthesized and characterized the DPP chromophores and the TEMPO-based catalyst. D.A.-G. and A.E. performed the DFT calculations and analysed the data. D.A.-G. and M.A.B. designed and characterized the alcohol oxidation photoanode, and carried out the PEC experiments of the photoanode. E.E.M. designed and characterized the CO2 reduction cathode. D.A.-G. and E.E.M. carried out the PEC experiments of the two-electrode cell, and analysed the data. A.R.O. and I.A.C.P. expressed, purified and characterized FDH. D.A.-G., J.W. and E.R. wrote the manuscript with contributions and discussions from all the authors. E.R. and J.W. supervised the research work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Synthesis thanks Wenzhen Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Alison Stoddart was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–16, Figs. 1–30 and References 1–11.
Source data
Source Data Fig. 2
Numerical data used to generate graphs.
Source Data Fig. 3
Numerical data used to generate graphs.
Source Data Fig. 4
Numerical data used to generate graphs.
Rights and permissions
About this article
Cite this article
Antón-García, D., Edwardes Moore, E., Bajada, M.A. et al. Photoelectrochemical hybrid cell for unbiased CO2 reduction coupled to alcohol oxidation. Nat Synth 1, 77–86 (2022). https://doi.org/10.1038/s44160-021-00003-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-021-00003-2
This article is cited by
-
Paired photoelectrochemical conversion of CO2/H2O and glycerol at high rate
Nature Catalysis (2024)
-
An allosteric redox switch involved in oxygen protection in a CO2 reductase
Nature Chemical Biology (2024)
-
Embedding biocatalysts in a redox polymer enhances the performance of dye-sensitized photocathodes in bias-free photoelectrochemical water splitting
Nature Communications (2024)
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
Syngas production by photoreforming of formic acid with 2D VxW1−xN1.5 solid solution as an efficient cocatalyst
Frontiers in Energy (2024)