Abstract
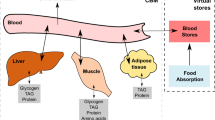
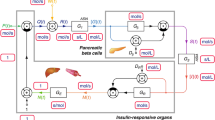
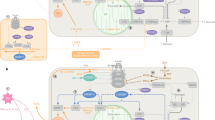
Type 1 diabetes (T1D) mellitus is a systemic disease triggered by a local autoimmune inflammatory reaction in insulin-producing cells that induce organ-wide, long-term metabolic effects. Mathematical modeling of the whole-body regulatory bihormonal system has helped to identify therapeutic interventions but is limited to a coarse-grained representation of metabolism. To extend the depiction of T1D, we developed a whole-body model of organ-specific regulation and metabolism that highlighted chronic inflammation as a hallmark of the disease, identified processes related to neurodegenerative disorders and suggested calcium channel blockers as adjuvants for diabetes control. In addition, whole-body modeling of a patient population allowed for the assessment of between-individual variability to insulin and suggested that peripheral glucose levels are degenerate biomarkers of the internal metabolic state. Taken together, the organ-resolved, dynamic modeling approach enables modeling and simulation of metabolic disease at greater levels of coverage and precision and the generation of hypothesis from a molecular level up to the population level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The male whole-body metabolic model10 is available at the Virtual Metabolic Human48 database (http://vmh.life), the glucose–insulin model6 is available at https://github.com/Open-Systems-Pharmacology/Glucose-Insulin-Model. Source data for Figs. 2–6 are provided with this paper and in Zenodo64.
Code availability
All related code is available at https://github.com/ThieleLab/dwbm and in Zenodo64.
References
Maahs, D. M., West, N. A., Lawrence, J. M. & Mayer-Davis, E. J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. North Am. 39, 481–497 (2010).
Orchard, T. J., Costacou, T., Kretowski, A. & Nesto, R. W. Type 1 diabetes and coronary artery disease. Diabetes Care 29, 2528–2538 (2006).
Thomas, N. J. et al. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 6, 122–129 (2018).
American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69 (2010).
Heinemann, L. Variability of insulin absorption and insulin action. Diabetes Technol. Ther. 4, 673–682 (2002).
Schaller, S. et al. A generic integrated physiologically based whole-body model of the glucose–insulin–glucagon regulatory system. CPT Pharmacomet. Syst. Pharm. 2, e65 (2013).
Wadehn, F., Schaller, S., Eissing, T., Krauss, M. & Küpfer, L. A multiscale, model-based analysis of the multi-tissue interplay underlying blood glucose regulation in type I diabetes. In Proc. 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE, 2016).
Schaller, S. et al. Robust PBPK/PD-based model predictive control of blood glucose. IEEE Trans. Biomed. Eng. 63, 1492–1504 (2016).
Lahoz-Beneytez, J. et al. Physiologically based simulations of deuterated glucose for quantifying cell turnover in humans. Front. Immunol. 8, 474 (2017).
Thiele, I. et al. Personalized whole-body models integrate metabolism, physiology, and the gut microbiome. Mol. Syst. Biol. 16, e8982 (2020).
Brunk, E. et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018).
Karr, J. R. et al. A whole-cell computational model predicts phenotype from genotype. Cell 150, 389–401 (2012).
Grafahrend-Belau, E. et al. Multiscale metabolic modeling: dynamic flux balance analysis on a whole-plant scale. Plant Physiol. 163, 637–647 (2013).
Krauss, M. et al. Integrating cellular metabolism into a multiscale whole-body model. PLoS Comput. Biol. 8, e1002750 (2012).
Cordes, H., Thiel, C., Baier, V., Blank, L. M. & Kuepfer, L. Integration of genome-scale metabolic networks into whole-body PBPK models shows phenotype-specific cases of drug-induced metabolic perturbation. NPJ Syst. Biol. Appl 4, 10 (2018).
Guebila, M. B. & Thiele, I. Model-based dietary optimization for late-stage, levodopa-treated, Parkinson’s disease patients. npj Syst. Biol. Appl. 2, 16013 (2016).
Thiele, I., Clancy, C. M., Heinken, A. & Fleming, R. M. T. Quantitative systems pharmacology and the personalized drug–microbiota–diet axis. Curr. Opin. Syst. Biol. 4, 43–52 (2017).
Yugi, K. et al. Reconstruction of insulin signal flow from phosphoproteome and metabolome data. Cell Rep. 8, 1171–1183 (2014).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Planas, R. et al. Gene expression profiles for the human pancreas and purified islets in type 1 diabetes: new findings at clinical onset and in long-standing diabetes. Clin. Exp. Immunol. 159, 23–44 (2010).
Mahadevan, R. & Schilling, C. H. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab. Eng. 5, 264–276 (2003).
Subramanian, A. et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 171, 1437–1452 e1417 (2017).
Banks, M. L., Hutsell, B. A., Blough, B. E., Poklis, J. L. & Negus, S. S. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int. J. Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyv009 (2015).
Sarkar, A. X. & Sobie, E. A. Regression analysis for constraining free parameters in electrophysiological models of cardiac cells. PLoS Comput. Biol. 6, e1000914 (2010).
Clayton, H. W. et al. Pancreatic inflammation redirects acinar to beta cell reprogramming. Cell Rep. 17, 2028–2041 (2016).
McCall, A. L. & Farhy, L. S. Treating type 1 diabetes: from strategies for insulin delivery to dual hormonal control. Minerva Endocrinol. 38, 145–163 (2013).
Ma, G., Allen, T. J., Cooper, M. E. & Cao, Z. Calcium channel blockers, either amlodipine or mibefradil, ameliorate renal injury in experimental diabetes. Kidney Int. 66, 1090–1098 (2004).
Lu, Y. et al. Mibefradil reduces blood glucose concentration in db/db mice. Clinics 69, 61–67 (2014).
Massry, S. G. & Smogorzewski, M. Role of elevated cytosolic calcium in the pathogenesis of complications in diabetes mellitus. Min. Electrolyte Metab. 23, 253–260 (1997).
Almaas, E., Kovacs, B., Vicsek, T., Oltvai, Z. N. & Barabasi, A. L. Global organization of metabolic fluxes in the bacterium Escherichia coli. Nature 427, 839–843 (2004).
Sahoo, S., Franzson, L., Jonsson, J. J. & Thiele, I. A compendium of inborn errors of metabolism mapped onto the human metabolic network. Mol. Biosyst. 8, 2545–2558 (2012).
Sargeant, R. J. & Paquet, M. R. Effect of insulin on the rates of synthesis and degradation of GLUT1 and GLUT4 glucose transporters in 3T3-L1 adipocytes. Biochem. J. 290, 913–919 (1993).
Bonora, E. & Tuomilehto, J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 34, S184–S190 (2011).
Gilarranz, L. J., Rayfield, B., Linan-Cembrano, G., Bascompte, J. & Gonzalez, A. Effects of network modularity on the spread of perturbation impact in experimental metapopulations. Science 357, 199–201 (2017).
Dromms, R. A., Lee, J. Y. & Styczynski, M. P. LK-DFBA: a linear programming-based modeling strategy for capturing dynamics and metabolite-dependent regulation in metabolism. BMC Bioinformatics 21, 93 (2020).
Goldenberg, J. Z. et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 372, m4743 (2021).
Lennerz, B. S., Koutnik, A. P., Azova, S., Wolfsdorf, J. I. & Ludwig, D. S. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J. Clin. Invest. https://doi.org/10.1172/JCI142246 (2021).
Akirov, A., Diker-Cohen, T., Masri-Iraqi, H. & Shimon, I. High glucose variability increases mortality risk in hospitalized patients. J. Clin. Endocrinol. Metab. 102, 2230–2241 (2017).
Tomkin, G. H. & Owens, D. Obesity diabetes and the role of bile acids in metabolism. J. Transl. Int. Med. 4, 73–80 (2016).
Correa-Giannella, M. L. & Machado, U. F. SLC2A4gene: a promising target for pharmacogenomics of insulin resistance. Pharmacogenomics 14, 847–850 (2013).
Sands, A. T. et al. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 38, 1181–1188 (2015).
Verma, S., McMurray, J. J. V. & Cherney, D. Z. I. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2, 939–940 (2017).
Gaster, M., Staehr, P., Beck-Nielsen, H., Schroder, H. D. & Handberg, A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes 50, 1324–1329 (2001).
Shan, W. F., Chen, B. Q., Zhu, S. J., Jiang, L. & Zhou, Y. F. Effects of GLUT4 expression on insulin resistance in patients with advanced liver cirrhosis. J. Zhejiang Univ. Sci. B 12, 677–682 (2011).
Richter, E. A. & Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 (2013).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Mauri, M., Elli, T., Caviglia, G., Uboldi, G. & Azzi, M. RAWGraphs: a visualisation platform to create open outputs. In Proc. 12th Biannual Conference on Italian SIGCHI Ch. 28 (ACM, 2017).
Noronha, A. et al. The Virtual Metabolic Human database: integrating human and gut microbiome metabolism with nutrition and disease. Nucleic Acids Res. 47, D614–D624 (2019).
Covert, M. W., Xiao, N., Chen, T. J. & Karr, J. R. Integrating metabolic, transcriptional regulatory and signal transduction models in Escherichia coli. Bioinformatics 24, 2044–2050 (2008).
Regittnig, W. et al. Plasma and interstitial glucose dynamics after intravenous glucose injection: evaluation of the single-compartment glucose distribution assumption in the minimal models. Diabetes 48, 1070–1081 (1999).
Sorensen, J. T. A Physiologic Model of Glucose Metabolism in Man and Its Use to Design and Assess Improved Insulin Therapies for Diabetes (Massachusetts Institute of Technology, 1985).
El-Khatib, F. H., Russell, S. J., Nathan, D. M., Sutherlin, R. G. & Damiano, E. R. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci. Transl. Med. 2, 27ra27 (2010).
Lewis, N. E. et al. Omic data from evolved E. coli are consistent with computed optimal growth from genome‐scale models. Mol. Syst. Biol. 6, 390 (2010).
Toroghi, M. K., Cluett, W. R. & Mahadevan, R. A multi-scale model of the whole human body based on dynamic parsimonious flux balance analysis. IFAC-PapersOnLine 49, 937–942 (2016).
Segre, D., Vitkup, D. & Church, G. M. Analysis of optimality in natural and perturbed metabolic networks. Proc. Natl Acad. Sci. USA 99, 15112–15117 (2002).
Heirendt, L. et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 14, 639–702 (2019).
Sobie, E. A. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys. J. 96, 1264–1274 (2009).
Gudmundsson, S. & Thiele, I. Computationally efficient flux variability analysis. BMC Bioinformatics 11, 489 (2010).
Thiele, I. & Palsson, B. Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 5, 93–121 (2010).
Duan, Q. et al. LINCS Canvas Browser: interactive web app to query, browse and interrogate LINCS L1000 gene expression signatures. Nucleic Acids Res. 42, W449–W460 (2014).
Mahadevan, R., Edwards, J. S. & Doyle, F. J. III Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J. 83, 1331–1340 (2002).
Heinemann, A., Wischhusen, F., Puschel, K. & Rogiers, X. Standard liver volume in the Caucasian population. Liver Transpl. Surg. 5, 366–368 (1999).
Bordbar, A. et al. Elucidating dynamic metabolic physiology through network integration of quantitative time-course metabolomics. Sci. Rep. 7, 46249 (2017).
Guebila, M. B. & Thiele, I. Dynamic flux balance analysis on whole-body metabolism for type 1 diabetes (version 0.2). Zenodo https://doi.org/10.5281/zenodo.4670413 (2021).
Acknowledgements
We acknowledge Bayer Technology Services for providing an academic version of the PK-SIM/MOBI software suite and the source file for the GIM model, K. Yugi from the University of Tokyo for providing the insulin model, and the Molecular Systems Physiology lab members at the University of Luxembourg for reviewing the manuscript and valuable discussions. Vector elements for Figs. 1, 4 and 6 were taken from vecteezy.com and freepik.com. This study was funded by Luxembourg National Research Fund (FNR) through the ATTRACT programme grant (FNR/A12/01, awarded to I.T.) and by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 757922, awarded to I.T.). The funders had no role in study design, data collection, data analysis, or interpretation.
Author information
Authors and Affiliations
Contributions
M.B.G. and I.T. conceived the study. M.B.G. developed the framework and carried out the simulations and analysis. M.B.G. and I.T. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Computational Science thanks Thomas Eissing, Laurence Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Handling editor: Ananya Rastogi, in collaboration with the Nature Computational Science team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, figures and tables.
Source data
Source Data Fig. 2
GIM simulation results from 0 to 600 min at 5 min intervals for 14 glucose and insulin challenges.
Source Data Fig. 3
Minimum and maximum bounds of healthy and T1D whole-body model with irreversible reactions.
Source Data Fig. 4
FVA solutions for the T1D model.
Source Data Fig. 5
Simulation results of 30 patients + 1 average patient for 600 min at 5 min time step.
Source Data Fig. 6
CRONICS simulation results for 30 patients from 16 min after insulin injection to 600 min at a time step of 2.5 min.
Rights and permissions
About this article
Cite this article
Ben Guebila, M., Thiele, I. Dynamic flux balance analysis of whole-body metabolism for type 1 diabetes. Nat Comput Sci 1, 348–361 (2021). https://doi.org/10.1038/s43588-021-00074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43588-021-00074-3
This article is cited by
-
A dynamic metabolic map for diabetes
Nature Computational Science (2021)