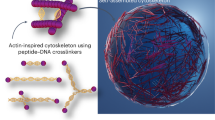
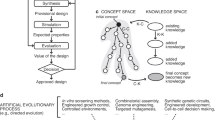
Abstract
Micropatterned materials have developed in nature over 3.8 billion years, with remarkable performance. Nature inspiration is the process of understanding biological mechanisms and strategies and using this knowledge to solve technical problems or enhance engineering designs. The term nature inspiration includes biomimetics, biomimicry and bioinspiration. Micropatterns are patterned materials or structures that have feature sizes in the nanometre-to-micrometre range. Owing to size effects, micropatterns can impart novel functions to a material without altering the bulk composition and properties. By imitating natural strategies, engineering challenges can be solved. Examples include mimicking ecological and in vivo microenvironments or producing superhydrophobic surfaces, adhesives and structural colours. Nature inspiration is an interdisciplinary field, involving various materials and fabrication techniques. This Primer provides a comprehensive overview of nature-inspired micropatterns, which offer unique design strategies and functional properties. In this Primer, key principles, fabrication methods and applications of nature-inspired micropatterns are discussed. Additionally, future prospects and challenges are highlighted for this rapidly evolving field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Patek, S. N. Biomimetics and evolution. Science 345, 1448–1449 (2014).
Broeckhoven, C. & du Plessis, A. Escaping the labyrinth of bioinspiration: biodiversity as key to successful product innovation. Adv. Funct. Mater. 32, 2110235 (2022).
Hetzler, B. E., Trauner, D. & Lawrence, A. L. Natural product anticipation through synthesis. Nat. Rev. Chem. 6, 170–181 (2022).
Ganewatta, M. S., Wang, Z. & Tang, C. Chemical syntheses of bioinspired and biomimetic polymers toward biobased materials. Nat. Rev. Chem. 5, 753–772 (2021).
Yang, J. et al. Biologically modified nanoparticles as theranostic bionanomaterials. Prog. Mater. Sci. 118, 100768 (2020).
Wegst, U., Bai, H., Saiz, E., Tomisa, A. P. & Ritchie, R. O. Bioinspired structural materials. Nat. Mater. 14, 23–36 (2015).
Sanchez, C., Arribart, H. & Giraud Guille, M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 4, 277–288 (2005).
Liu, M., Wang, S. & Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2, 17036 (2017).
Darmanin, T. & Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 18, 273–285 (2015).
Xiang, T. et al. Biomimetic micro/nano structures for biomedical applications. Nano Today 35, 100980 (2020).
Yoo, J. W., Irvine, D. J., Discher, D. E. & Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 10, 521–535 (2011).
Proppe, A. H. et al. Bioinspiration in light harvesting and catalysis. Nat. Rev. Mater. 5, 828–846 (2020).
Heuer, A. H. et al. Innovative materials processing strategies: a biomimetic approach. Science. 255, 1098–1105 (1992).
Wang, Y. et al. Bioinspired structural color patch with anisotropic surface adhesion. Sci. Adv. 6, eaax8258 (2020).
Zhang, B. et al. Advanced bio-inspired structural materials: local properties determine overall performance. Mater. Today 41, 177–199 (2020).
ISO/TC266 2015 Biomimetics — Terminology, Concepts and Methodology, ISO 18458 (Beuth, 2015).
Fayemi, P. E., Wanieck, K., Zollfrank, C., Maranzana, N. & Aoussat, A. Biomimetics: process, tools and practice. Bioinspir. Biomim. 12, 011002 (2017). This paper summarized some terms about ‘learning from nature’, for example, bionic, bioinspire, biomimetic and biomimicry. The definition, the common features and the differences are compared.
Katiyar, N. K., Goel, G., Hawi, S. & Goel, S. Nature-inspired materials: emerging trends and prospects. NPG Asia Mater. 13, 56 (2021).
Barnes, W. J. P. Biomimetic solutions to sticky problems. Science 318, 203–204 (2007).
Lei, Z., Zhu, W., Zhang, X., Wang, X. & Wu, P. Bio-inspired ionic skin for theranostics. Adv. Funct. Mater. 31, 2008020 (2021).
Xu, X. et al. Improving plant photosynthesis through light-harvesting upconversion nanoparticles. ACS Nano 16, 18027–18037 (2022).
Varenberg, M. & Gorb, S. N. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 21, 483–486 (2009).
Liimatainen, V., Drotlef, D. M., Son, D. & Sitti, M. Liquid-superrepellent bioinspired fibrillar adhesives. Adv. Mater. 32, 2000497 (2020).
Bažant, Z. P. Size effect. Int. J. Solids Struct. 37, 69–80 (2000).
Boesel, L. F. et al. Gecko-inspired surfaces: a path to strong and reversible dry adhesives. Adv. Mater. 22, 2125–2137 (2010). This paper described the impact of ‘size effect’ on the adhesive properties of micropattern.
Zhao, Y., Xie, Z., Gu, H., Zhu, C. & Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 41, 3297–3317 (2012).
Saleh, T. A. Nanomaterials: classification, properties, and environmental toxicities. Environ. Technol. Innov. 20, 101067 (2020). This review summarized the mechanisms of size effect.
Banik, A. & Tan, K. T. Dynamic friction performance of hierarchical biomimetic surface pattern inspired by frog toe-pad. Adv. Mater. Interfaces 7, 2000987 (2020).
Iturri, J. et al. Torrent frog-inspired adhesives: attachment to flooded surfaces. Adv. Funct. Mater. 25, 1499–1505 (2015).
Meng, F. et al. Tree frog-inspired structured hydrogel adhesive with regulated liquid. Adv. Mater. Interfaces 8, 2100528 (2021).
Geim, A. et al. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2, 461–463 (2003).
Wang, D. et al. Sensing-triggered stiffness-tunable smart adhesives. Sci. Adv. 9, eadf4051 (2023).
Ruotolo, W., Brouwer, D. & Cutkosky, M. R. From grasping to manipulation with gecko-inspired adhesives on a multifinger gripper. Sci. Robot. 6, eabi9773 (2021).
Jiang, H. et al. A robotic device using gecko-inspired adhesives can grasp and manipulate large objects in microgravity. Sci. Robot. 2, eaan4545 (2017).
Hu, S. et al. Liquid repellency enhancement through flexible microstructures. Sci. Adv. 6, eaba9721 (2020).
Li, Y., Liu, B. F. & Zhang, X. Wettability-patterned microchip for emerging biomedical materials and technologies. Mater. Today 51, 273–293 (2021).
Wang, F., Wu, Y. & Nestler, B. Wetting effect on patterned substrate. Adv. Mater. 35, 2210745 (2023).
Huang, F. et al. Wetting-enhanced structural color for convenient and reversible encryption of optical information. ACS Appl. Mater. Interfaces 13, 42276–42286 (2021). This paper summarized theories about wetting effects on patterned substrates, with emphasis on mathematical models.
Kolle, M. et al. Mimicking the colourful wing scale structure of the Papilio blumei butterfly. Nat. Nanotechnol. 5, 511–515 (2010).
Davis, A. L., Nijhout, H. F. & Johnsen, S. Diverse nanostructures underlie thin ultra-black scales in butterflies. Nat. Commun. 11, 1294 (2020).
Gu, Y. et al. Three-dimensional transistor arrays for intra- and inter-cellular recording. Nat. Nanotechnol. 17, 292–300 (2022).
Wang, Y. et al. Microenvironment-controlled micropatterned microfluidic model (MMMM) for biomimetic in situ studies. ACS Nano 14, 9861–9872 (2020).
Han, S. et al. A digital microfluidic diluter-based microalgal motion biosensor for marine pollution monitoring. Biosens. Bioelectron. 143, 111597 (2019).
Wang, X. et al. Differentiation of bMSCs on biocompatible, biodegradable, and biomimetic scaffolds for largely defected tissue repair. ACS Appl. Bio Mater 3, 735–746 (2019).
Schaffner, M., England, G., Kolle, M., Aizenberg, J. & Vogel, N. Combining bottom-up self-assembly with top-down microfabrication to create hierarchical inverse opals with high structural order. Small 11, 4334–4340 (2015).
Egan, P., Sinko, R., LeDuc, P. R. & Keten, S. The role of mechanics in biological and bio-inspired systems. Nat. Commun. 6, 7418 (2015).
Zhang, X. et al. Polyphenol and self-assembly: metal polyphenol nanonetwork for drug delivery and pharmaceutical applications. Future Drug Discov. 1, FDD7 (2019).
Dumanli, A. G. & Savin, T. Recent advances in the biomimicry of structural colours. Chem. Soc. Rev. 45, 6698–6724 (2016).
Baumeister, D., Tocke, R., Dwyer, J., Ritter, S. & Benyus, J. Biomimicry resource handbook: a seed bank of best practices. Biomimicry 3, 286 (2014).
Bandyopadhyay, P. R., Hrubes, J. D. & Leinhos, H. A. Biorobotic adhesion in water using suction cups. Bioinspir. Biomim. 3, 016003 (2008).
Baik, S., Kim, J., Lee, H. J., Lee, T. H. & Pang, C. Highly adaptable and biocompatible octopus-like adhesive patches with meniscus-controlled unfoldable 3D microtips for underwater surface and hairy skin. Adv. Sci. 5, 1800100 (2018).
Baik, S. et al. A wet-tolerant adhesive patch inspired by protuberances in suction cups of octopi. Nature 546, 396–400 (2017).
Chen, Y. et al. Controlled growth of patterned conducting polymer microsuckers on superhydrophobic micropillar-structured templates. Adv. Funct. Mater. 28, 1800240 (2018).
Penick, C. A. et al. The comparative approach to bio-inspired design: integrating biodiversity and biologists into the design process. Integr. Comp. Biol. 62, 1153–1163 (2022).
Ronaldson-Bouchard, K. et al. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 6, 351–371 (2022).
Zheng, G. et al. Development of microfluidic dilution network-based system for lab-on-a-chip microalgal bioassays. Anal. Chem. 90, 13280–13289 (2018).
Pan, Y., Yang, Z., Li, C., Hassan, S. U. & Shum, H. C. Plant-inspired TransfOrigami microfluidics. Sci. Adv. 8, eabo1719 (2022).
Wanieck, K., Fayemi, P., Maranzana, N., Zollfrank, C. & Jacobs, S. Biomimetics and its tools. Bioinspir. Biomim. Nanobiomater. 6, 53–66 (2017). This paper gives an overview of more than 40 existing tools, which have been developed to facilitate and support biomimetics.
Houten, F. et al. Bio-based design methodologies for products, processes, machine tools and production systems. CIRP J. Manuf. Sci. Technol. 32, 46–60 (2021).
Scarangella, A., Soldan, V. & Mitov, M. Biomimetic design of iridescent insect cuticles with tailored, self-organized cholesteric patterns. Nat. Commun. 11, 4108 (2020).
Cheng, X. et al. Programming 3D curved mesosurfaces using microlattice designs. Science 379, 1225–1232 (2023).
He, X. et al. Prediction of the lotus effect on solid surfaces by machine learning. Small 18, 2203264 (2022).
Lill, T. & Joubert, O. The cutting edge of plasma etching. Science 319, 1050–1051 (2008).
Kustandi, T. S. et al. Self-assembled nanoparticles based fabrication of gecko foot-hair-inspired polymer nanofibers. Adv. Funct. Mater. 17, 2211–2218 (2007).
Huang, Y. F. et al. Improved broadband and quasi-omnidirectional anti-reflection properties with biomimetic silicon nanostructures. Nat. Nanotech. 2, 770–774 (2007).
Wu, L. et al. Toward controllable wet etching of monocrystalline silicon: roles of mechanically driven defects. ACS Appl. Mater. Interfaces 14, 29366–29376 (2022).
Guo, D. J. et al. Fabrication and adhesion of a bio-inspired microarray: capillarity-induced casting using porous silicon mold. J. Mater. Chem. B 1, 379–386 (2013).
Xue, L. et al. Hybrid surface patterns mimicking the design of the adhesive toe pad of tree frog. ACS Nano 11, 9711–9719 (2017).
Xue, L. et al. Humidity-enhanced wet adhesion on insect-inspired fibrillar adhesive pads. Nat. Commun. 6, 6621 (2015).
Yi, H. et al. Wet-responsive, reconfigurable, and biocompatible hydrogel adhesive films for transfer printing of nanomembranes. Adv. Funct. Mater. 28, 1706498 (2018).
Yi, H., Lee, S. H., Seong, M., Kwak, M. K. & Jeong, H. E. Bioinspired reversible hydrogel adhesives for wet and underwater surfaces. J. Mater. Chem. B 6, 8064–8070 (2018).
Minchenkov, K., Vedernikov, A., Safonov, A. & Akhatov, I. Thermoplastic pultrusion: a review. Polymers 13, 180 (2021).
Gorb, S., Varenberg, M., Peressadko, A. & Tuma, J. Biomimetic mushroom-shaped fibrillar adhesive microstructure. J. R. Soc. Interface 4, 271–275 (2007).
Bormashenko, E., Stein, T., Pogreb, R. & Aurbach, D. “Petal effect” on surfaces based on lycopodium: high-stick surfaces demonstrating high apparent contact angles. J. Phys. Chem. C 113, 5568–5572 (2009).
Zhang, E., Liu, Y., Yu, J., Lv, T. & Li, L. Fabrication of hierarchical gecko-inspired microarrays using a three-dimensional porous nickel oxide template. J. Mater. Chem. B 3, 6571–6575 (2015).
Drotlef, D. M. et al. Insights into the adhesive mechanisms of tree frogs using artificial mimics. Adv. Funct. Mater. 23, 1137–1146 (2013).
Shakeri, A., Khan, S. & Didar, T. F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 21, 3053–3075 (2021).
Yuk, H., Zhang, T., Parada, G. A., Liu, X. & Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 7, 12028 (2016).
Huang, R. et al. Suction cups-inspired adhesive patch with tailorable patterns for versatile wound healing. Adv. Sci. 8, 2100201 (2021).
Jin, K. et al. Biomimetic bidirectional switchable adhesive inspired by the gecko. Adv. Funct. Mater. 24, 574–579 (2014).
Seong, M., Park, H. H., Hwang, I. & Jeong, H. E. Strong and reversible adhesion of interlocked 3D-microarchitectures. Coatings 9, 48 (2019).
Kim, D. W. et al. Highly permeable skin patch with conductive hierarchical architectures inspired by amphibians and octopi for omnidirectionally enhanced wet adhesion. Adv. Funct. Mater. 29, 1807614 (2019).
Reddy, S., Arzt, E. & del Campo, A. Bioinspired surfaces with switchable adhesion. Adv. Mater. 19, 3833–3837 (2007).
Yoo, P. J. et al. Unconventional patterning with a modulus-tunable mold: from imprinting to microcontact printing. Chem. Mater. 16, 5000–5005 (2004).
Xiang, Y. et al. Superrepellency of underwater hierarchical structures on Salvinia leaf. Proc. Natl Acad. Sci. USA 117, 2282–2287 (2020).
Wang, Q. et al. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 463, 339–343 (2010).
Guan, Q. F. et al. Sustainable cellulose-nanofiber based hydrogels. ACS Nano 15, 7889–7898 (2021).
Davidson, M. D. et al. Mechanochemical adhesion and plasticity in multifiber hydrogel networks. Adv. Mater. 32, 1905719 (2020).
Li, Z. et al. Nature-derived bionanomaterials for sustained release of 5-fluorouracil to inhibit subconjunctival fibrosis. Mater. Today Adv. 11, 100150 (2021).
Ouyang, J. et al. In situ sprayed NIR-responsive, analgesic black phosphorus-based gel for diabetic ulcer treatment. Proc. Natl Acad. Sci. USA 117, 28667–28677 (2020).
Tang, H. et al. In situ forming epidermal bioelectronics for daily monitoring and comprehensive exercise. ACS Nano 16, 17931–17947 (2022).
Jin, L. et al. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact. Mater. 16, 162–172 (2022).
Aisenbrey, E. A. & Murphy, W. L. Synthetic alternative to Matrigel. Nat. Rev. Mater. 5, 539–551 (2020).
Chowdhury, M. S. et al. Dendronized fluorosurfactant for highly stable water-in-fluorinated oil emulsions with minimal inter-droplet transfer of small molecules. Nat. Commun. 10, 4546 (2019).
Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017). This paper reviews the development of hydrogels.
Richbourg, N. R. et al. Precise control of synthetic hydrogel network structure via linear, independent synthesis–swelling relationships. Sci. Adv. 7, eabe3245 (2021).
Ruan, H. et al. Engineered extracellular vesicles for ischemic stroke treatment. Innovation 4, 100394 (2023).
Zou, W. et al. Cytocompatible chitosan based multi-network hydrogels with antimicrobial, cell anti-adhesive and mechanical properties. Carbohydr. Polym. 202, 246–257 (2018).
Rao, P. et al. Tough hydrogels with fast, strong, and reversible underwater adhesion based on a multiscale design. Adv. Mater. 30, e1801884 (2018).
Daly, A. C., Prendergast, M. E., Hughes, A. J. & Burdick, J. A. Bioprinting for the biologist. Cell 184, 18–32 (2021).
Ma, Y. et al. Remote control over underwater dynamic attachment/detachment and locomotion. Adv. Mater. 30, 1801595 (2018).
Wu, Q. et al. Bio-inspired active self-cleaning surfaces via filament-like sweepers array. Adv. Mater. 35, 2212246 (2023).
Alameda, M. T., Osorio, M. R., Hernández, J. J. & Rodríguez, I. Multilevel hierarchical topographies by combined photolithography and nanoimprinting processes to create surfaces with controlled wetting. ACS Appl. Nano Mater. 2, 4727–4733 (2019).
Linares, A. V., Falcimaigne-Cordin, A., Gheber, L. A. & Haupt, K. Patterning nanostructured, synthetic, polymeric receptors by simultaneous projection photolithography, nanomolding, and molecular imprinting. Small 7, 2318–2325 (2011).
Akamatsu, K., Samitsu, S., Tsuruoka, T., Hasegawa, J. & Nawafune, H. Site-selective integration of monolayer-protected inorganic nanoparticles onto surface monolayer templates by a solvent-induced lift-off process. Small 2, 1130–1133 (2006).
Liao, W. S. et al. Subtractive patterning via chemical lift-off lithography. Science 337, 1517–1521 (2012).
Adelung, R. et al. Strain-controlled growth of nanowires within thin-film cracks. Nat. Mater. 3, 375–379 (2004).
Xue, L. et al. Tailoring normal adhesion of arrays of thermoplastic, spring-like polymer nanorods by shaping nanorod tips. Langmuir 28, 10781–10788 (2012).
Riesgo, B. et al. Effect of hydrofluoric acid concentration and etching time on the adhesive and mechanical behavior of glass-ceramics: a systematic review and meta-analysis. Int. J. Adhes. Adhes. 121, 103303 (2023).
Sun, H. et al. Etching of two-dimensional materials. Mater. Today 42, 192–213 (2021).
Han, J. et al. Anisotropic wet etching silicon tips of small opening angle in KOH solution with the additions of I2/KI. Sens. Actuators A: Phys. 152, 75–79 (2009).
Li, W. et al. An adhesive bioink toward biofabrication under wet conditions. Small https://doi.org/10.1002/smll.202205078 (2023).
Wei, Y., Wang, F. & Guo, Z. Bio-inspired and metal-derived superwetting surfaces: function, stability and applications. Adv. Colloid Interface Sci. 314, 102879 (2023).
Schmidt, O. & Eberl, K. Thin solid films roll up into nanotubes. Nature 410, 168 (2001).
Sen, A. K., Raj, A., Banerjee, U. & Iqbal, S. R. in Microfluidic Electrophoresis: Methods and Protocols (ed. Dutta, D.) 13–54 (Springer, 2019).
Swarup, S. D. & Arjyajyoti, G. Hot embossing of polymers — a review. Mater. Today: Proc. 26, 405–414 (2020).
Kim, J. K. et al. Enhanced flexible mold lifetime for roll-to-roll scaled-up manufacturing of adhesive complex microstructures. Adv. Mater. 35, e2207257 (2023).
Han, F. et al. Triple-synergistic 2D material-based dual-delivery antibiotic platform. NPG Asia Mater. 12, 15 (2020).
Ji, X. et al. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat. Commun. 12, 1124 (2021).
Wang, S. et al. Machine-learning micropattern manufacturing. Nano Today 38, 101152 (2021).
Zheng, G. et al. Microfluidic chemostatic bioreactor for high-throughput screening and sustainable co-harvesting of biomass and biodiesel in microalgae. Bioact. Mater. 25, 629–639 (2023).
Ouyang, J. et al. A facile and general method for synthesis of antibiotic-free protein-based hydrogel: wound dressing for the eradication of drug-resistant bacteria and biofilms. Bioact. Mater. 18, 446–458 (2022).
Papautsky, I. & Peterson, E.T.K. in Micromolding: Encyclopedia of Microfluidics and Nanofluidics (ed. Li, D.) (Springer, 2014).
Li, Q. et al. Replica molding-based nanopatterning of tribocharge on elastomer with application to electrohydrodynamic nanolithography. Nat. Commun. 9, 974 (2018).
Xu, Q. et al. Biomimetic selfcleaning surfaces: synthesis, mechanism and applications. J. R. Soc. Interface 13, 20160300 (2016).
Li, X., Tian, J. & Shen, W. Progress in patterned paper sizing for fabrication of paper-based microfluidic sensors. Cellulose 17, 649–659 (2010).
Wang, H. et al. Paper-based three-dimensional microfluidic device for monitoring of heavy metals with a camera cell phone. Anal. Bioanal. Chem. 406, 2799–2807 (2014).
Wang, M. et al. A three-dimensional pinwheel-shaped paper-based microfluidic analytical device for fluorescence detection of multiple heavy metals in coastal waters by rational device design. Anal. Bioanal. Chem. 413, 3299–3313 (2021).
Bruzewicz, D. A., Reches, M. & Whitesides, G. M. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Anal. chem. 80, 3387–3392 (2008).
Xia, H. et al. Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuators B 312, 127962 (2020).
Kotz, F. et al. Fused deposition modeling of microfluidic chips in polymethylmethacrylate. Micromachines 11, 873 (2020).
Gross, B. C. et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 86, 3240–3253 (2014).
Liao, J. et al. 3D-printable colloidal photonic crystals. Mater. Today 56, 29–41 (2022).
Cui, H. et al. 4D physiologically adaptable cardiac patch: a 4-month in vivo study for the treatment of myocardial infarction. Sci. Adv. 6, eabb5067 (2020).
Li, H. et al. Scattered seeding of CAR T cells in solid tumors augments anticancer efficacy. Natl Sci. Rev. 9, nwab172 (2022).
Tricinci, O., Pignatelli, F. & Mattoli, V. 3D micropatterned functional surface inspired by Salvinia molesta via direct laser lithography for air retention and drag reduction. Adv. Funct. Mater. https://doi.org/10.1002/adfm.202206946 (2023).
Brown, P. S. & Bhushan, B. Designing bioinspired superoleophobic surfaces. APL Mater. 4, 015703 (2016). This paper explained the physical mechanisms about contact angle of droplet on the micropatterned surfaces. Wenzel state and Cassie–Baxter state are briefly summarized.
Hensel, R., Moh, K. & Arzt, E. Engineering micropatterned dry adhesives: from contact theory to handling applications. Adv. Funct. Mater. 28, 1800865 (2018).
Chen, H. et al. Bioinspired surface for surgical graspers based on the strong wet friction of tree frog toe pads. ACS Appl. Mater. Interfaces 7, 13987–13995 (2015).
Vogel, M. J. & Steen, P. H. Capillarity-based switchable adhesion. Proc. Natl Acad. Sci. USA 107, 3377–3381 (2010).
Tvon Erlach, T. C. et al. Cell-geometry-dependent changes in plasma membrane order direct stem cell signalling and fate. Nat. Mater. 17, 237–242 (2018).
Théry, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl Acad. Sci. USA 103, 19771–19776 (2006).
Zheng, G. et al. An integrated microfluidic device in marine microalgae culture for toxicity screening application. Mar. Pollut. Bull. 72, 231–243 (2013).
Ahmed, T., Shimizu, T. S. & Stocker, R. Microfluidics for bacterial chemotaxis. Integr. Biol. 2, 604–629 (2010).
Englert, D. L., Manson, M. D. & Jayaraman, A. Flow-based microfluidic device for quantifying bacterial chemotaxis in stable, competing gradients. Appl. Environ. Microbiol. 75, 4557–4564 (2009).
Durham, W. M., Tranzer, O., Leombruni, A. & Stocker, R. Division by fluid incision: biofilm patch development in porous media. Phys. Fluids 24, 091107 (2012).
Marcos & Stocker, R. Microorganisms in vortices: a microfluidic setup. Limnol. Oceanogr. Methods 4, 392–398 (2006).
Li, F. et al. Bioresource upgrade for sustainable energy, environment, and biomedicine. Nano-Micro Lett. 15, 35 (2023).
Han, B. et al. Computer-aided design of microfluidic resistive network using circuit partition and CFD-based optimization and application in microalgae assessment for marine ecological toxicity. Bioprocess Biosyst. Eng. 42, 785–797 (2019).
Feng, C. Y. et al. An on-chip pollutant toxicity determination based on marine microalgal swimming inhibition. Analyst 141, 1761–1771 (2016).
Zheng, G. et al. A microfluidic droplet array demonstrating high-throughput screening in individual lipid-producing microalgae. Anal. Chim. Acta 1227, 340322 (2022).
Mehrabi, Z., Slade, E. M., Solis, A. & Mann, D. J. The importance of microhabitat for biodiversity sampling. PLoS ONE 9, e114015 (2014).
Wang, Y. et al. An integrated digital microfluidic bioreactor for fully automatic screening of microalgal growth and stress-induced lipid accumulation. Biotechnol. Bioeng. 118, 294–304 (2021).
Brumley, D. R. et al. Bacteria push the limits of chemotactic precision to navigate dynamic chemical gradients. Proc. Natl Acad. Sci. USA 116, 10792–10797 (2019).
Smriga, S., Fernandez, V. I., Mitchell, J. G. & Stocker, R. Chemotaxis toward phytoplankton drives organic matter partitioning among marine bacteria. Proc. Natl Acad. Sci. USA 113, 1576–1581 (2016).
Dörgens, A. L. et al. A laboratory model of marine snow: preparation and characterization of porous fiber particles. Limnol. Oceanogr. Methods 13, 664–671 (2015).
Shapiro, O. H., Kramarsky-Winter, E., Gavish, A. R., Stocker, R. & Vardi, A. A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat. Commun. 7, 10860 (2016).
Long, T. & Ford, R. M. Enhanced transverse migration of bacteria by chemotaxis in a porous T-sensor. Environ. Sci. Technol. 435, 1546–1552 (2008).
Marcos, Fu, H. C., Powers, T. R. & Stocker, R. Bacterial rheotaxis. Proc. Natl Acad. Sci. USA 109, 4780–4785 (2012).
Lauga, E., DiLuzio, W. R., Whitesides, G. M. & Stone, H. A. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90, 400–412 (2006).
DiLuzio, W. R. et al. Escherichia coli swim on the right-hand side. Nature 435, 1271–1274 (2005).
Perera-Costa, D., Bruque, J. M., González-Martín, M. L., Gómez-García, A. C. & Vadillo-Rodríguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 30, 4633–4641 (2014).
White, A. R., Jalali, M. & Sheng, J. A new ecology-on-a-chip microfluidic platform to study interactions of microbes with a rising oil droplet. Sci. Rep. 9, 13737 (2019).
Pașca, S. P. et al. A nomenclature consensus for nervous system organoids and assembloids. Nature 609, 907–910 (2022).
Lancaster, M. A. & Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 (2014).
Zhou, J. et al. Biomaterials and nanomedicine for bone regeneration: progress and future prospects. Exploration 1, 20210011 (2021).
Castillo Ransanz, L., Van Altena, P. F. J., Heine, V. M. & Accardo, A. Engineered cell culture microenvironments for mechanobiology studies of brain neural cells. Front. Bioeng. Biotech. 10, 1096054 (2022).
Chen, X., Zhang, Y. S., Zhang, X. & Liu, C. Organ-on-a-chip platforms for accelerating the evaluation of nanomedicine. Bioact. Mater. 6, 1012–1027 (2021).
Koyilot, M. C. et al. Breakthroughs and applications of organ-on-a-chip technology. Cells 11, 1828 (2022).
Zhang, B., Korolj, A., Lai, B. F. L. & Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 3, 257–278 (2018).
Zheng, G.-X. et al. An integrated microfluidic device for culturing and screening of Giardia lamblia. Exp. Parasitol. 137, 1–7 (2014).
Lu, L. et al. Measurement of Giardia lamblia adhesion force using an integrated microfluidic assay. Anal. Bioanal. Chem. 409, 1451–1459 (2017).
Markus, J. et al. Human small intestinal organotypic culture model for drug permeation, inflammation, and toxicity assays. In Vitro Cell. Dev. Anim. 57, 160–173 (2021).
Velasco, V., Soucy, P., Keynton, R. & Williams, S. J. A microfluidic impedance platform for real-time, in vitro characterization of endothelial cells undergoing fluid shear stress. Lab on a Chip 22, 4705–4716 (2022).
Damkham, N., Issaragrisil, S. & Lorthongpanich, C. Role of YAP as a mechanosensing molecule in stem cells and stem cell-derived hematopoietic cells. Int. J. Mol. Sci. 23, 14634 (2022).
Fletcher, D. & Mullins, R. Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010).
Wang, L. et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540, 579–582 (2016).
Baik, S. et al. Bioinspired adhesive architectures: from skin patch to integrated bioelectronics. Adv. Mater. 31, e1803309 (2019).
Sarkar, N., Bhumiratana, S., Geris, L., Papantoniou, I. & Grayson, W. L. Bioreactors for engineering patient-specific tissue grafts. Nat. Rev. Bioeng. 1, 361–377 (2023).
Matai, I., Kaur, G., Seyedsalehi, A., McClinton, A. & Laurencin, C. T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226, 119536 (2020).
Manfrin, A. et al. Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 16, 640–648 (2019).
Britton, G., Chhabra, S., Massey, J. & Warmflash, A. in Programmed Morphogenesis: Methods and Protocols (eds Ebrahimkhani, M. R. & Hislop, J.) 119–130 (Humana Press, 2021).
Stronati, E. et al. YAP1 regulates the self-organized fate patterning of hESC-derived gastruloids. Stem Cell Rep. 17, 211–220 (2022).
Ashammakhi, N. et al. Advancing frontiers in bone bioprinting. Adv. Healthcare Mater. 8, 1801048 (2019).
Daly, A. C. et al. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthcare Mater. 6, 1700298 (2017).
Vijayavenkataraman, S., Lu, W. F. & Fuh, J. Y. H. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 8, 032001 (2016).
Ma, X. et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl Acad. Sci. USA 113, 2206–2211 (2016).
Knowlton, S., Anand, S., Shah, T. & Tasoglu, S. Bioprinting for neural tissue engineering. Trends Neurosci. 41, 31–46 (2018).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotech. 32, 773–785 (2014).
Moroni, L. et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 3, 21–37 (2018).
Derakhshanfar, S. et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 3, 144–156 (2018).
Ahadian, S. & Khademhosseini, A. A perspective on 3D bioprinting in tissue regeneration. Bio-Des. Manuf. 1, 157–160 (2018).
Li, H. et al. Handheld bioprinting strategies for in situ wound dressing. Essays Biochem. 65, 533–543 (2021).
Ashammakhi, N. et al. In situ three-dimensional printing for reparative and regenerative therapy. Biomed. Microdev. 21, 42 (2019).
Wu, Y., Ravnic, D. J. & Ozbolat, I. T. Intraoperative bioprinting: repairing tissues and organs in a surgical setting. Trends Biotechnol. 38, 594–605 (2020).
Eremeyev, V. A. in Encyclopedia of Continuum Mechanics (eds Altenbach, H. & Öchsner, A.) 2290–2291 (Springer, 2020).
Ruiz-Esparza, G. U. et al. Nanoengineered shear-thinning hydrogel barrier for preventing postoperative abdominal adhesions. Nano-Micro Lett. 13, 212 (2021).
Tian, H. et al. Core–shell dry adhesives for rough surfaces via electrically responsive self-growing strategy. Nat. Commun. 13, 7659 (2022).
Hu, S. & Xia, Z. Rational design and nanofabrication of gecko-inspired fibrillar adhesives. Small 8, 2464–2468 (2012).
Chen, C. M., Chiang, C. L., Lai, C. L., Xie, T. & Yang, S. Buckling-based strong dry adhesives via interlocking. Adv. Funct. Mater. 23, 3813–3823 (2013).
Zhu, M., Zhang, F. & Chen, X. Bioinspired mechanically interlocking structures. Small Struct. 1, 2000045 (2020).
Yuk, H. et al. Dry double-sided tape for adhesion of wet tissues and devices. Nature 575, 169–174 (2019).
Chen, Y. et al. Bioinspired multiscale wet adhesive surfaces: structures and controlled adhesion. Adv. Funct. Mater. 30, 1905287 (2020).
Huebsch, N. & Mooney, D. J. Inspiration and application in the evolution of biomaterials. Nature 462, 426–432 (2009).
Autumn, K. et al. Gecko adhesion as a model system for integrative biology, interdisciplinary science, and bioinspired engineering. Annu. Rev. Ecol. Evol. Syst. 45, 445–470 (2014).
Bartlett, M. D. et al. Looking beyond fibrillar features to scale gecko-like adhesion. Adv. Mater. 24, 1078–1083 (2012).
Aksak, B. et al. Adhesion of biologically inspired vertical and angled polymer microfiber arrays. Langmuir 23, 3322–3332 (2007).
Qu, L. et al. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 322, 238–242 (2008).
Gillies, A. G. et al. Dry self-cleaning properties of hard and soft fibrillar structures. ACS Appl. Mater. Interfaces 5, 6081–6088 (2013).
Xu, Q. et al. Robust self-cleaning and micromanipulation capabilities of gecko spatulae and their bio-mimics. Nat. Commun. 6, 8949 (2015).
Murphy, M. P. et al. Gecko-inspired directional and controllable adhesion. Small 5, 170–175 (2009).
Lee, H., Lee, B. P. & Messersmith, P. B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341 (2007).
Huber, G. et al. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl Acad. Sci. USA 102, 16293–16296 (2005).
Zhang, C. et al. Hydraulically coupled dielectric elastomer actuators for a bioinspired suction cup. Polymers 13, 3481 (2021).
Blossey, R. Self-cleaning surfaces — virtual realities. Nat. Mater. 2, 301–306 (2003).
Bhushan, B., Jung, Y. C. & Koch, K. Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philos. Trans. R. Soc. A 367, 1631–1672 (2009).
Koch, K., Bhushan, B. & Barthlott, W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 4, 1943–1963 (2008).
Fürstner, R., Barthlott, W., Neinhuis, C. & Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 21, 956–961 (2005).
Wang, D. et al. Design of robust superhydrophobic surfaces. Nature 582, 55–59 (2020).
Toma, M., Loget, G. & Corn, R. M. Flexible teflon nanocone array surfaces with tunable superhydrophobicity for self-cleaning and aqueous droplet patterning. ACS Appl. Mater. Interfaces 6, 11110–11117 (2014).
Gao, X. & Jiang, L. Water-repellent legs of water striders. Nature 432, 36–36 (2004).
Yang, Y. et al. 3D-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 30, 1704912 (2018).
Liu, T. L. & Kim, C. J. C. Turning a surface superrepellent even to completely wetting liquids. Science 346, 1096–1100 (2014).
Bohn, H. F. & Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl Acad. Sci. USA 101, 14138–14143 (2004).
Deng, X., Mammen, L., Butt, H. J. & Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 335, 67–70 (2012).
Tuteja, A., Choi, W., Mabry, J. M., McKinley, G. H. & Cohen, R. E. Robust omniphobic surfaces. Proc. Natl Acad. Sci. USA 105, 18200–18205 (2008).
Saison, T. et al. Replication of butterfly wing and natural lotus leaf structures by nanoimprint on silica sol–gel films. Bioinspir. Biomim. 3, 046004 (2008).
Liu, Y., Chen, X. & Xin, J. H. Hydrophobic duck feathers and their simulation on textile substrates for water repellent treatment. Bioinspir. Biomim. 3, 046007 (2008).
Gan, Z., Turner, M. D. & Gu, M. Biomimetic gyroid nanostructures exceeding their natural origins. Sci. Adv. 2, e1600084 (2016).
Shen, Q. et al. Subtractive structural modification of morpho butterfly wings. Small 11, 5705–5711 (2015).
Li, C. & Qi, L. Colloidal-crystal-assisted patterning of crystalline materials. Adv. Mater. 22, 1494–1497 (2010).
Yang, S. M., Jang, S. G., Choi, D. G., Kim, S. & Yu, H. K. Nanomachining by colloidal lithography. Small 2, 458–475 (2006).
Wang, F., Zhang, X., Lin, Y., Wang, L. & Zhu, J. Structural coloration pigments based on carbon modified ZnS@SiO2 nanospheres with low-angle dependence, high color saturation, and enhanced stability. ACS Appl. Mater. Interfaces 8, 5009–5016 (2016).
England, G. T. et al. The optical Janus effect: asymmetric structural color reflection materials. Adv. Mater. 29, 1606876 (2017).
Fu, F., Shang, L., Chen, Z., Yu, Y. & Zhao, Y. Bioinspired living structural color hydrogels. Sci. Rob. 3, eaar8580 (2018).
Goerlitzer, E. S. A., Klupp Taylor, R. N. & Vogel, N. Bioinspired photonic pigments from colloidal self-assembly. Adv. Mater. 30, 1706654 (2018).
Bogatyrev, N. & Bogatyreva, O. in Eco-Innovation and the Development of Business Models: Lessons from Experience and New Frontiers in Theory and Practice (eds Garrido Azevedo, S., Brandenburg, M., Carvalho, H. & Cruz-Machado, V.) 297–314 (Springer International Publishing, 2014).
Vincent, J. F. V., Bogatyreva, O. A., Bogatyrev, N. R., Bowyer, A. & Pahl, A. K. Biomimetics: its practice and theory. J. R. Soc. Interface 3, 471–482 (2006).
Abdala, L. N., Fernandes, R. B., Ogliari, A., Löwer, M. & Feldhusen, J. Creative contributions of the methods of inventive principles of TRIZ and BioTRIZ to problem solving. ASME J. Mech. Des. 139, 082001 (2017).
Badarnah, L. & Kadri, U. A methodology for the generation of biomimetic design concepts. Architect. Sci. Rev. 58, 120–133 (2015).
Imani, N. & Vale, B. A framework for finding inspiration in nature: biomimetic energy efficient building design. Energy Build. 225, 110296 (2020).
Leung, C. M. et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2, 33 (2022).
Fang, G., Chen, Y. C., Lu, H. & Jin, D. Advances in spheroids and organoids on a chip. Adv. Funct. Mater. 33, 2215043(2023).
Liu, L. et al. Artificial intelligence-powered microfluidics for nanomedicine and materials synthesis. Nanoscale 13, 19352–19366 (2021).
Wang, B. et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence. Nat. Commun. 14, 1341 (2023).
Wang, X., Xie, P., Chen, B. & Zhang, X. Chip-based high-dimensional optical neural network. Nano-Micro Lett. 14, 221 (2022).
Yang, Z. et al. Thermal immuno-nanomedicine in cancer. Nat. Rev. Clin. Oncol. 20, 116–134 (2023).
Yang, L. et al. Diffusion models: a comprehensive survey of methods and applications. Preprint at https://doi.org/10.48550/arXiv.2209.00796 (2022).
Mahdavi, A. et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl Acad. Sci. USA 105, 2307–2312 (2008).
Acknowledgements
X.Z. specially acknowledges the love and support from grandparents W. Zhang and Y. Qiu and MIT Professor M. Dresselhaus.
Author information
Authors and Affiliations
Contributions
Introduction (Y.W., G.Z., M.G., Y.S.Z. and X.Z.); Experimentation (Y.W., G.Z., N.J., G.Y., Y.S.Z. and X.Z.); Results (Y.W., G.Z., J.M., Y.S.Z. and X.Z.); Applications (Y.W., G.Z., L.M., Y.S.Z. and X.Z.); Reproducibility and data deposition (Y.W., G.Z., X.C., Y.S.Z. and X.Z.); Limitations and optimizations (Y.W., G.Z., Y.S.Z. and X.Z.); Outlook (Y.W., G.Z., Y.L., X.C., M.G., Y.S.Z. and X.Z.); and overview of the Primer (all authors).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Mehmet Sarikaya and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AskNature: https://asknature.org/
Glossary
- Biomimetic
-
Biomimetics is the interdisciplinary cooperation of biology and technology to solve practical problems through functional analysis of biological systems, abstraction into models and application to a solution.
- Bioinspiration
-
A creative approach based on the observation of biological systems, extraction of functional principles and use of those principles in engineered systems. Although a biomimetic design must imitate its natural counterpart, bioinspiration is influenced or informed by a biological system.
- Material islands
-
Small, isolated regions or patches of a material that are uniquely patterned on the substrate surface.
- Micro-electromechanical systems
-
(MEMS). Integrated mechanical elements, sensors, actuators and electronics that are fabricated using microfabrication technologies derived from the semiconductor industry.
- Surface wettability
-
The character of a surface that allows liquids to adhere. Surface wettability can be described by contact angles (CAs), the tangent angle between the solid surface and liquid meniscus. When CA > 90°, the liquid tends to form a droplet on the surface, and the surface is hydrophobic. Conversely, when CA < 90°, the liquids tend to spread on the surface and the surface is hydrophilic.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Zheng, G., Jiang, N. et al. Nature-inspired micropatterns. Nat Rev Methods Primers 3, 68 (2023). https://doi.org/10.1038/s43586-023-00251-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00251-w
This article is cited by
-
Intelligent Vascularized 3D/4D/5D/6D-Printed Tissue Scaffolds
Nano-Micro Letters (2023)