Abstract
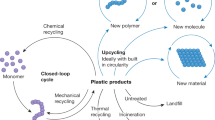
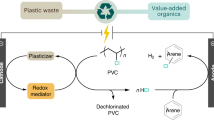
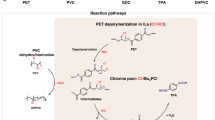
Over the past decade, chlorinated plastics have been widely used as an indispensable thermoplastic owing to their low cost, durability, wide processing adaptability and good overall performance in plenty of end-use applications. One effective pathway to reduce plastic white pollution is to upcycle chlorinated plastics for added value and versatility rather than recycle them. This Primer focuses on upcycling technologies for converting chlorinated waste plastics into additional valuable products and endowing them with added versatility. We describe several upcycling strategies for the conversion of chlorinated waste plastics into value-added products, which involve pretreatment to reduce the chlorine content; pyrolysis, carbonization or catalytic cracking; or chemical modifications such as substitution with functional groups and plasticizers, and grafting with other polymers. Additionally, solvent-based processing is discussed, including solvent extraction, and dissolution, gel casting and solvothermal treatments are also included. This Primer aims to stimulate both research and industry to produce high-quality and high-value chemicals from upcycled chlorinated plastics that are suitable for value-added manufacture to provide the necessary environmental and economic push in the context of carbon neutrality and sustainable development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhao, X., et al. Upcycling to sustainably reuse plastics. Adv. Mater. 34, 2100843 (2022).
Chen, X., Wang, Y. & Zhang, L. Recent progress in the chemical upcycling of plastic wastes. ChemSusChem 14, 4137–4151 (2021).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Skolia, E., Mountanea, O. G. & Kokotos, C. G. Photochemical upcycling of polystyrene plastics. Trends Chem. 5, 116–120 (2022).
Wang, C., Kelley, S. S. & Venditti, R. A. Lignin‐based thermoplastic materials. ChemSusChem 9, 770–783 (2016).
Jiang, G., Sanchez Monsalve, D., Clough, P., Jiang, Y. & Leeke, G. A. Understanding the dechlorination of chlorinated hydrocarbons in the pyrolysis of mixed plastics. ACS Sustain. Chem. Eng. 9, 1576–1589 (2021).
Li, N., et al. Conversion of plastic waste into fuels: a critical review. J. Hazard. Mater. 424, 127460 (2022).
Liu, Y., Zhou, C., Li, F., Liu, H. & Yang, J. Stocks and flows of polyvinyl chloride (PVC) in China: 1980–2050. Resour. Conserv. Recycl. 154, 104584 (2020).
International Council of Chemical Associations. UNEA-5 and A Global Framework to End Plastic Waste (ICCA, 2023).
Zhang, G. et al. Room-temperature rapid synthesis of metal-free doped carbon materials. Carbon 115, 28–33 (2017).
Chang, Y. et al. Polymer dehalogenation-enabled fast fabrication of N,S-codoped carbon materials for superior supercapacitor and deionization applications. ACS Appl. Mater. Interfaces 9, 29753–29759 (2017).
Xu, C., Wang, S., Shao, L., Zhao, J. & Feng, Y. Structure and properties of chlorinated polyvinyl chloride graft copolymer with higher property. Polym. Adv. Technol. 23, 470–477 (2012).
Ma, Y., et al. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration—still on the run. React. Funct. Polym. 147, 104458 (2020).
Begum S. A., Rane, A. V. & Kanny, K. in Applications of compatibilized polymer blends in automobile industry (eds Ajitha, A. R. & Sabu, T.) 563–593 (Elsevier, 2020).
Mathew, J., Joy, J. & George, S. C. Potential applications of nanotechnology in transportation: a review. J. King Saud. Univ. Sci. 31, 586–594 (2019).
Grand View Research. PVC Packaging Market Size, Share & Trends Analysis Report by Product (Durable, Non-durable), by Application (Food & Beverage, Pharmaceuticals, Cosmetics & Personal Care), by Region, and Segment Forecasts, 2021–2028 (Grand View Research, 2021).
Data Bridge Market Research. Global Polyvinyl Chloride (PVC) Market Report. DBMR https://www.databridgemarketresearch.com/reports/global-polyvinyl-chloride-pvc-market (2022).
Zion Market Research. Global PVC Market Set for Rapid Growth, to Reach Around USD 78.90 Billion by 2021 (GlobeNewswire, 2016).
Patrick, S. Practical Guide to Polyvinyl Chloride (iSmithers Rapra, 2005).
Chanda, M. & Roy, S. K. Industrial Polymers, Specialty Polymers, and Their Applications (CRC, 2008).
Rahimi, A. & García, J. M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 1, 0046 (2017).
Iles, A., Martin, A. & Rosen, C. M. in Ethics of Chemistry: From Poison Gas to Climate Engineering (eds Schummer, J. & Borsen, T.) 113–316 (World Scientific, 2021).
Cruz, P. P. R., Da Silva, L. C., Fiuza-Jr, R. A. & Polli, H. Thermal dehydrochlorination of pure PVC polymer: part I — thermal degradation kinetics by thermogravimetric analysis. J. Appl. Polym. Sci. 138, 50598 (2021).
Liu, P., Chen, W., Liu, Y., Bai, S. & Wang, Q. Thermal melt processing to prepare halogen-free flame retardant poly(vinyl alcohol). Polym. Degrad. Stab. 109, 261–269 (2014).
Tullo, A. H. Plasticizer makers want a piece of the phthalates pie. Chem. Eng. News 93, 16–18 (2015).
Zedan, H. Red List of Threatened Species: A Global Species Assessment (IUCN, 2004).
Zhang, F. et al. From trash to treasure: chemical recycling and upcycling of commodity plastic waste to fuels, high-valued chemicals and advanced materials. J. Energy Chem. 69, 369–388 (2022).
Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021). This work discusses closed-loop recycling of PE plastics through depolymerization, solvolysis, common recycled moulding and polycondensation of long-chain building blocks derived from plant oil feedstocks or microalgal oils using cutting-edge catalytic techniques.
Lee, A. & Liew, M. S. Tertiary recycling of plastics waste: an analysis of feedstock, chemical and biological degradation methods. J. Mater. Cycles Waste Manag. 23, 32–43 (2021).
Wang, M.-M., Zhang, C.-C. & Zhang, F.-S. Recycling of spent lithium-ion battery with polyvinyl chloride by mechanochemical process. Waste Manag. 67, 232–239 (2017).
Kameda, T., Wachi, S., Grause, G., Mizoguchi, T. & Yoshioka, T. Dehydrochlorination of poly(vinyl chloride) with Ca(OH)2 in ethylene glycol and the effect of ball milling. J. Polym. Res. 18, 1687–1691 (2011).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022). This work presents a perspective on the upcycling of plastic waste into specialized polymers, fine chemicals and new materials with added value for a sustainable plastics economy.
Hou, Q. et al. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2, 100514 (2021).
Duangchan, A. & Samart, C. Tertiary recycling of PVC-containing plastic waste by copyrolysis with cattle manure. Waste Manag. 28, 2415–2421 (2008).
Organisation for Economic Co-operation and Development (OECD). Global plastics outlook database. OECD https://www.oecd-ilibrary.org/environment/data/global-plastic-outlook_c0821f81-en (2023).
Hundertmark, T., Mayer, M., McNally, C., Simons, T. J. & Witte, C. How plastics waste recycling could transform the chemical industry. McKinsey & Company https://www.mckinsey.com/industries/chemicals/our-insights/how-plastics-waste-recycling-could-transform-the-chemical-industry#/ (2018).
Miliute-Plepiene, J., Fråne, A. & Almasi, A. M. Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol. 4, 100246 (2021).
Glas, D., Hulsbosch, J., Dubois, P., Binnemans, K. & Devos, D. E. End-of-life treatment of poly(vinyl chloride) and chlorinated polyethylene by dehydrochlorination in ionic liquids. ChemSusChem 7, 610–617 (2014).
Wang, H. et al. Solid base assisted dual-promoted heterogeneous conversion of PVC to metal-free porous carbon catalyst. Chem. Eur. J. 28, e202200124 (2022). This work presents a solvent and solid-base dual-promoted solvothermal method for converting PVC into high-value chemicals at a relatively low temperature (<200 °C), which has the potential to inexpensively recover PVC with low chlorinated content and high conversion yields for commercial application.
Zhou, Q. et al. Lanthania promoted MgO: simultaneous highly efficient catalytic degradation and dehydrochlorination of polypropylene/polyvinyl chloride. Appl. Catal. B. 80, 141–146 (2008).
Jiao, X. et al. Photocatalytic conversion of waste plastics into C2 fuels under simulated natural environment conditions. Angew. Chem. Int. Ed. 59, 15497–15501 (2020). This work presents a novel photo-induced C–C cleavage and coupling route for converting chlorinated waste polymers into C2 chemicals under normal external conditions.
Jia, P. et al. Cardanol groups grafted on poly (vinyl chloride) — synthesis, performance and plasticization mechanism. Polymers 9, 621 (2017).
Beveridge, J. M., Chenot, H. M., Crich, A., Jacob, A. & Finn, M. Covalent functionalization of flexible polyvinyl chloride tubing. Langmuir 34, 10407–10412 (2018).
Lee, K. W., Chung, J. W. & Kwak, S.-Y. Structurally enhanced self‐plasticization of poly(vinyl chloride) via click grafting of hyperbranched polyglycerol. Macromol. Rapid Commun. 37, 2045–2051 (2016).
Kim, J. K., Lee, C. S., Lee, S.-Y., Cho, H. H. & Kim, J. H. Bimodal porous TiO2 structures templated by graft copolymer/homopolymer blend for dye-sensitized solar cells with polymer electrolyte. J. Power Sources 336, 286–297 (2016).
Chi, W. S. et al. Direct organization of morphology-controllable mesoporous SnO2 using amphiphilic graft copolymer for gas-sensing applications. ACS Appl. Mater. Interface 9, 37246–37253 (2017).
Park, M. S., Walsh, D., Zhang, J., Kim, J. H. & Eslava, S. Efficient hematite photoanodes prepared by hydrochloric acid-treated solutions with amphiphilic graft copolymer. J. Power Sources 404, 149–158 (2018).
Gates, B. C. Catalytic Chemistry (Wiley, 1991).
Zinovyev, S., Perosa, A., Yufit, S. & Tundo, P. Hydrodechlorination and hydrogenation over Raney–Ni under multiphase conditions: role of multiphase environment in reaction kinetics and selectivity. J. Catal. 211, 347–354 (2002).
Assefa, M. K. & Fieser, M. E. Divergent silylium catalysis enables facile poly (vinyl chloride) upcycling to poly(ethylene-co-styrene) derivatives. J. Mater. Chem. A. 11, 2128–2132 (2023). This recent report highlights the ability to turn PVC into polyethylene-co-styrene using an in situ-generated silylium catalyst.
Wu, Y.-H., et al. Poly(ethylene glycol) enhanced dehydrochlorination of poly(vinyl chloride). J. Hazard. Mater. 163, 1408–1411 (2009).
Fagnani, D. E., Kim, D., Camarero, S. I., Alfaro, J. F. & Mcneil, A. J. Using waste poly(vinyl chloride) to synthesize chloroarenes by plasticizer-mediated electro (de)chlorination. Nat. Chem. 15, 222–229 (2023). This recent paper highlights how waste PVC can be used as a chlorine source in place of HCl, using electrocatalytic methods.
Shapoval, G., Tomilov, A., Pud, A. & Batsalova, K. The electrochemical reductive degradation of polyvinyl chloride. Polym. Sci. 29, 1564–1572 (1987).
Greco, A., Ferrari, F. & Maffezzoli, A. UV and thermal stability of soft PVC plasticized with cardanol derivatives. J. Clean. Prod. 164, 757–764 (2017).
Laukaitienė, A., et al. Investigation of polyvinyl chloride and thermoplastic polyurethane waste blend miscibility. Mater. Sci. 19, 397–402 (2013).
Elshekeil, Y., Sapuan, S., Jawaid, M. & Al-Shujaa, O. Influence of fiber content on mechanical, morphological and thermal properties of kenaf fibers reinforced poly(vinyl chloride)/thermoplastic polyurethane poly-blend composites. Mater. Des. 58, 130–135 (2014).
Brookman, R. S. A new PVC based polymer for medical applications. J. Vinyl Addit. Technol. 13, 191–194 (1991).
Teyssie, P. & Smets, G. Polymers and group interactions. II. Friedel–Crafts reactions on polyvinyl chloride, a route to poly‐1,3‐methyleneindans. J. Polym. Sci. 20, 351–369 (1956). This work shows how Friedel–Crafts reactions can graft styrenic groups onto the polymer backbone of PVC (and release HCl), which is one of the first examples of chemical substitution on the PVC backbone.
Kabir, G. & Hameed, B. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sust. Energ. Rev. 70, 945–967 (2017).
Harvey, A. N., Snape, I. & Siciliano, S. D. Validating potential toxicity assays to assess petroleum hydrocarbon toxicity in polar soil. Environ. Toxicol. Chem. 31, 402–407 (2012).
Corwin, D. L. in Encyclopedia of Water Science (eds Stewart, B. A. & Howell, T.) 4–7 (CRC press, 2003).
Li, J., Cai, J., Yuan, T., Guo, H. & Qi, F. A thermal decomposition study of polymers by tunable synchrotron vacuum ultraviolet photoionization mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1269–1274 (2009).
Moulay, S. Chemical modification of poly(vinyl chloride) — still on the run. Prog. Polym. Sci. 35, 303–331 (2010).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 114, 2708–2711 (2002).
Fantoni, N. Z., El-Sagheer, A. H. & Brown, T. A hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 121, 7122–7154 (2021).
Liang, L. & Astruc, D. The copper (I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 255, 2933–2945 (2011).
Earla, A., Li, L., Costanzo, P. & Braslau, R. Phthalate plasticizers covalently linked to PVC via copper-free or copper catalyzed azide–alkyne cycloadditions. Polymer 109, 1–12 (2017).
Öztürk, T., Meyvacı, E. & Arslan, T. Synthesis and characterization of poly(vinyl chloride-g-ε-caprolactone) brush type graft copolymers by ring-opening polymerization and “click” chemistry. J. Macromol. Sci. A 57, 171–180 (2020).
Baxter, I. Sustainable Polymers: Upcycling Poly(Vinyl Chloride) and Mimicking Low Density Polyethylene (Univ. of South Carolina, 2022).
Thornton, J. Environmental Impacts of Polyvinyl Chloride (PVC) Building Materials (Healthy Building Network, 2002).
Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 8, 100023 (2021).
Zhou, S., Liu, C. & Zhang, L. Critical review on the chemical reaction pathways underpinning the primary decomposition behavior of chlorine-bearing compounds under simulated municipal solid waste incineration conditions. Energy Fuel 34, 1–15 (2019).
Chen, Y. et al. Catalytic dechlorination and charring reaction of polyvinyl chloride by CuAl layered double hydroxide. Energy Fuels 32, 2407–2413 (2018).
Bae, S. Y., et al. Dehydrochlorination of polyvinylchloride using Al-modified graphitic-C3N4. RSC Adv. 6, 20728–20733 (2016).
Dai, M. et al. Microwave-assisted fast co-pyrolysis behaviors and products between microalgae and polyvinyl chloride. Appl. Therm. Eng. 136, 9–15 (2018).
Dwivedi, P., Mishra, P., Mondal, M. K. & Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 5, e02198 (2019).
Zhou, N. et al. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 418, 129412 (2021).
Gopinath, K. P., Nagarajan, V. M., Krishnan, A. & Malolan, R. A critical review on the influence of energy, environmental and economic factors on various processes used to handle and recycle plastic wastes: development of a comprehensive index. J. Clean. Prod. 274, 123031 (2020).
Anikeeva, I., et al. Synthesis of carbon materials via mechanically activated dehydrochlorination of polyvinyl chloride. Russ. J. Appl. Chem. 91, 1830–1834 (2018).
Kryazhev, Y. G. et al. Porous carbon–carbon composite materials obtained by alkaline dehydrochlorination of polyvinyl xhloride. Materials 15, 7636 (2022).
Cheng, L. X., Zhang, L., Chen, X. Y. & Zhang, Z. J. Efficient conversion of waste polyvinyl chloride into nanoporous carbon incorporated with MnOx exhibiting superior electrochemical performance for supercapacitor application. Electrochim. Acta. 176, 197–206 (2015).
Sheng, Z. et al. Green synthesis of nitrogen-doped hierarchical porous carbon nanosheets derived from polyvinyl chloride towards high-performance supercapacitor. J. Power Sources 515, 230629 (2021).
Chang, Y. et al. Converting polyvinyl chloride plastic wastes to carbonaceous materials via room-temperature dehalogenation for high-performance supercapacitor. ACS Appl. Energy Mater. 1, 5685–5693 (2018).
Zhang, N. & Shen, Y. One-step pyrolysis of lignin and polyvinyl chloride for synthesis of porous carbon and its application for toluene sorption. Bioresour. Technol. 284, 325–332 (2019).
Min, J. et al. A general approach towards carbonization of plastic waste into a well-designed 3D porous carbon framework for super lithium-ion batteries. Chem. Commun. 56, 9142–9145 (2020).
Kobayashi, N., Inden, Y. & Endo, M. Silicon/soft-carbon nanohybrid material with low expansion for high capacity and long cycle life lithium-ion battery. J. Power Sources 326, 235–241 (2016).
Zhang, Z., Zhou, D., Zhou, L., Yu, H. & Huang, B. NiFe LDH-CoPc/CNTs as novel bifunctional electrocatalyst complex for zinc-air battery. Ionics 24, 1709–1714 (2018).
Cho, M.-H., Choi, Y.-K. & Kim, J.-S. Air gasification of PVC (polyvinyl chloride)-containing plastic waste in a two-stage gasifier using Ca-based additives and Ni-loaded activated carbon for the production of clean and hydrogen-rich producer gas. Energy 87, 586–593 (2015).
Li, Q., et al. Facile preparation of polydimethylsiloxane/carbon nanotubes modified melamine solar evaporators for efficient steam generation and desalination. J. Colloid Interface Sci. 584, 602–609 (2021).
Tian, C. et al. Sandwich photothermal membrane with confined hierarchical carbon cells enabling high-efficiency solar steam generation. Small 16, 2000573 (2020).
Gong, F. F. et al. Scalable, eco-friendly and ultrafast solar steam generators based on one-step melamine-derived carbon sponges toward water purification. Nano Energy 58, 322–330 (2019).
Gao, Y., Zhao, H., Chen, D., Chen, C. & Ciucci, F. In situ synthesis of mesoporous manganese oxide/sulfur-doped graphitized carbon as a bifunctional catalyst for oxygen evolution/reduction reactions. Carbon 94, 1028–1036 (2015).
Wang, J. et al. Polyvinyl chloride-derived carbon spheres for CO2 adsorption. ChemSusChem 13, 6426–6432 (2020).
Qian, M., Chen, I.-W. & Huang, F. Solar activated crude oil cleanup using net-shape-formed ultralight graphene tiles. Appl. Mater. Today 19, 100551 (2020).
Qian, M. et al. Ultra-light graphene tile-based phase-change material for efficient thermal and solar energy harvest. ACS Appl. Energy Mater. 3, 5517–5522 (2020).
Lu, L.,et al. A new strategy for CO2 utilization with waste plastics: conversion of hydrogen carbonate into formate using polyvinyl chloride in water. Green Chem. 22, 352–358 (2020).
Wang, L., Zhang, R.-Z., Deng, R. & Luo, Y.-H. Oxygen-induced enhancement in low-temperature dechlorination of PVC: an experimental and DFT study on the oxidative pyrolysis process. ACS Sustain. Chem. Eng. 9, 2835–2843 (2021).
Peng, C. et al. Low temperature co-pyrolysis of food waste with PVC-derived char: products distributions, char properties and mechanism of bio-oil upgrading. Energy 219, 119670 (2021).
Seino, A., Nishizaki, T., Kanda, Y., Sugioka, M. & Uemichi, Y. Development of chlorine tolerant degradation catalyst for chemical recycling of polyethylene. J. Jpn. Pet. Inst. 52, 70–71 (2009).
Aguado, J., Serrano, D., Sotelo, J., Van Grieken, R. & Escola, J. M. Influence of the operating variables on the catalytic conversion of a polyolefin mixture over HMCM-41 and nanosized HZSM-5. Ind. Eng. Chem. Res. 40, 5696–5704 (2001).
Uekert, T., Kasap, H. & Reisner, E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst. J. Am. Chem. Soc. 141, 15201–15210 (2019).
Zang, X., Dong, Y., Jian, C., Ferralis, N. & Grossman, J. C. Upgrading carbonaceous materials: coal, tar, pitch, and beyond. Matter 5, 430–447 (2022).
Lü, X. et al. Low-temperature rapid synthesis of high-quality pristine or boron-doped graphene via Wurtz-type reductive coupling reaction. J. Mater. Chem. 21, 10685–10689 (2011).
Weng, B., Wu, Z., Li, Z. & Yang, H. Hydrogen generation from hydrolysis of NH3BH3/MH (M = Li, Na) binary hydrides. Int. J. Hydrog. Energy 37, 5152–5160 (2012).
Lin, T., Huang, F., Liang, J. & Wang, Y. A facile preparation route for boron-doped graphene, and its CdTe solar cell application. Energy Environ. Sci. 4, 862–865 (2011).
Yoshioka, T., Furukawa, K. & Okuwaki, A. Chemical recycling of rigid-PVC by oxygen oxidation in NaOH solutions at elevated temperatures. Polym. Degrad. Stab. 67, 285–290 (2000).
Inoue, T., Miyazaki, M., Kamitani, M., Kano, J. & Saito, F. Dechlorination of polyvinyl chloride by its grinding with KOH and NaOH. Adv. Powder Technol. 16, 27–34 (2005).
Zhao, P., Li, T., Yan, W. & Yuan, L. Dechlorination of PVC wastes by hydrothermal treatment using alkaline additives. Environ. Technol. 39, 977–985 (2018).
Ma, D. et al. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process. Saf. Environ. 146, 108–117 (2021).
Fuentes, J. A. et al. On the functional group tolerance of ester hydrogenation and polyester depolymerization catalyzed by ruthenium complexes of tridentate aminophosphine ligands. Chem. Eur. J. 21, 10851–10860 (2015).
Lu, X., Ma, X., Chen, X., Yao, Z. & Zhang, C. Co-hydrothermal carbonization of polyvinyl chloride and corncob for clean solid fuel production. Bioresour. Technol. 301, 122763 (2020).
Yao, Z. & Ma, X. Characteristics of co-hydrothermal carbonization on polyvinyl chloride wastes with bamboo. Bioresour. Technol. 247, 302–309 (2018).
Ma, D., et al. Insight into chlorine evolution during hydrothermal carbonization of medical waste model. J. Hazard. Mater. 380, 120847 (2019).
Hashimoto, K., Suga, S., Wakayama, Y. & Funazukuri, T. Hydrothermal dechlorination of PVC in the presence of ammonia. J. Mater. Sci. 43, 2457–2462 (2008).
Lieberzeit, P., Bekchanov, D. & Mukhamediev, M. Polyvinyl chloride modifications, properties, and applications. Adv. Polym. Technol. 33, 1809–1820 (2022).
Queiroz, A., Pedroso, G. B., Kuriyama, S. N. & Fidalgo-Neto, A. A. Subcritical and supercritical water for chemical recycling of plastic waste. Curr. Opin. Green. Sustain. Chem. 25, 100364 (2020).
Čolnik, M., Kotnik, P., Knez, Ž. & Škerget, M. Degradation of polyvinyl chloride (PVC) waste with supercritical water. Processes 10, 1940 (2022).
Jia, C., et al. Applications, treatments, and reuse of plastics from electrical and electronic equipment. J. Ind. Eng. Chem. 110, 84–99 (2022).
Yu, C.-H. et al. Design strategies, surface functionalization, and environmental remediation potentialities of polymer-functionalized nanocomposites. Chemosphere 306, 135656 (2022).
Yan, B., et al. Review on porous carbon materials engineered by ZnO templates: design, synthesis and capacitance performance. Mater. Des. 201, 109518 (2021).
Xu, S. M., Liang, X., Ren, Z. C., Wang, K. X. & Chen, J. S. Free-standing air cathodes based on 3D hierarchically porous carbon membranes: kinetic overpotential of continuous macropores in Li–O2 batteries. Angew. Chem. Int. Ed. 130, 6941–6945 (2018).
Oster, K., et al. Dehydrochlorination of PVC in multi-layered blisterpacks using ionic liquids. Green. Chem. 22, 5132–5142 (2020).
Zhao, T. et al. A highly efficient approach for dehydrochlorinating polyvinyl chloride: catalysis by 1-butyl-3-methylimidazolium chloride. Green. Chem. 12, 1062–1065 (2010).
Klöpffer, W. The critical review of life cycle assessment studies according to ISO 14040 and 14044. Int. J. LCA 17, 1087–1093 (2012).
Serajiantehrani, R. Development of a Machine Learning-based Prediction Model for Construction and Environmental Costs of Trenchless Spray-applied Pipe Linings, Cured-in-Place Pipe, and Slip Lining Methods in Large Diameter Culverts (Univ. of Texas at Arlington, 2019).
Larosa, A. et al. Life cycle assessment of a novel hybrid glass-hemp/thermoset composite. J. Clean. Prod. 44, 69–76 (2013).
Mark, G. SimaPro 9.1.1 (PRé Sustainability, 2020).
Galvez-Martos, J.-L. & Schoenberger, H. An analysis of the use of life cycle assessment for waste co-incineration in cement kilns. Resour. Recycl. 86, 118–131 (2014).
Yung, K. C., Chan, C. & Zhang, Y. Application Guideline for Embedded GHG Emissions Database and G-BOM Analyzer (Computer Science, 2015).
Rosenbaum, R. K. et al. USEtox—the UNEP-SETAC toxicity model: recommended characterization factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. LCA 13, 532–546 (2008).
Dudek, A. Z., Arodz, T. & Gálvez, J. Computational methods in developing quantitative structure-activity relationships (QSAR): a review. Comb. Chem. High. Throughput Screen. 9, 213–228 (2006).
Cronin, M. T. in Recent advances in QSAR studies. Challenges and advances in computational chemistry and physics (eds Puzyn, T., Leszczynski, J. & Cronin, M.) 3–11 (Springer, 2010).
Nolte, T. M., Peijnenburg, W. J., Hendriks, A. J. & van de-Meent, D. Quantitative structure–activity relationships for green algae growth inhibition by polymer particles. Chemosphere 179, 49–56 (2017).
Tait, M. W. & Cheung, W. M. A comparative cradle-to-gate life cycle assessment of three concrete mix designs. Int. J. LCA 21, 847–860 (2016).
Yung, W. K., Muthu, S. S. & Subramanian, K. in Carbon Footprint Analysis of Printed Circuit Board (ed. Subramanian, S.) 365–431 (Butterworth-Heinemann, 2018).
Jiménez-González, C., Kim, S. & Overcash, M. R. Methodology for developing gate-to-gate life cycle inventory information. Int. J. LCA 5, 153–159 (2000).
Mohanty, A. K. et al. Sustainable polymers. Nat. Rev. Methods Primers 2, 1–27 (2022).
Yang, M., Zhao, P., Cui, X., Geng, F. & Guo, Q. Kinetics study on hydrothermal dechlorination of poly(vinyl chloride) by in-situ sampling. Environ. Technol. Innov. 23, 101703 (2021).
Jia, P., et al. Self-plasticization of PVC materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustain. Chem. Eng. 5, 6665–6673 (2017).
Noorani, N. & Mehrdad, A. Modification of PVC with 1-vinylimidazole for CO2/CH4 separation: sorption, permeation and DFT studies. Phys. Chem. Res. 8, 689–703 (2020).
Vancsó, G., Nagy, T., Turcsányi, B., Kelen, T. & Tüdós, F. Formation of fixed paramagnetic solitons in a polyacetylene analogue polymer matrices: ESR studies in thermally decomposed PVC. J. Phys. Colloq. 44, 703–704 (1983).
Li, T., Zhao, P., Lei, M. & Li, Z. Understanding hydrothermal dechlorination of PVC by focusing on the operating conditions and hydrochar characteristics. Appl. Sci. 7, 256 (2017).
Lin, Y.-H., Tseng, C.-C., Wei, T.-T. & Hsu, C.-T. Recycling of dual hazardous wastes in a catalytic fluidizing process. Catal. Today 174, 37–45 (2011).
Chen, X., et al. Fabrication of a high water flux conductive MWCNTs/PVC composite membrane with effective electrically enhanced antifouling behavior. Coatings 11, 1548 (2021).
Wang, C., Song, X., Liu, Y. & Zhang, C. PVC-g-PVP amphiphilic polymer synthesis by ATRP and its membrane separation performance for silicone-containing wastewater. Polymer 229, 123965 (2021).
Matsumoto, M., Takemori, S. & Tahara, Y. Lactic acid permeation through deep eutectic solvents-based polymer inclusion membranes. Membranes 10, 244 (2020).
Zhang, M. et al. A hybrid fibers based wearable fabric piezoelectric nanogenerator for energy harvesting application. Nano Energy 13, 298–305 (2015).
Sil, D. & Chakrabarti, S. Photocatalytic degradation of PVC–ZnO composite film under tropical sunlight and artificial UV radiation: a comparative study. Sol. Energy 84, 476–485 (2010).
Lin, H. et al. Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 film. J. Biomed. Mater. Res. B 87, 425–431 (2008).
Hwang, T., Frank, Z., Neubauer, J. & Kim, K. J. High-performance polyvinyl chloride gel artificial muscle actuator with graphene oxide and plasticizer. Sci. Rep. 9, 9658 (2019).
Hines, L., Petersen, K., Lum, G. Z. & Sitti, M. Soft actuators for small-scale robotics. Adv. Mater. 29, 1603483 (2017).
Taghavi, M., Chen, H. Y., Conn, A. T. & Rossiter, J. Stiffness graded electroactive artificial muscle. Adv. Funct. Mater. 32, 2200994 (2022).
Dong, T.-Y., Zhang, X.-I. & Liu, T. Artificial muscles for wearable assistance and rehabilitation. Front. Inf. Technol. Electron. Eng. 19, 1303–1315 (2018).
Li, Y., & Hashimoto, M. A proposal of a light-weight walking assist wear using PVC gel artificial muscles. in IEEE Int. Conf. Robotics and Automation 2920–2925 (IEEE, 2015).
Li, Y., Li, Y. & Hashimoto, M. Low-voltage planar PVC gel actuator with high performances. Sens. Actuators B Chem. 282, 482–489 (2019).
Gent, A. & Shimizu, N. Elasticity, tear strength, and strength of adhesion of soft PVC gels. J. Appl. Polym. Sci. 32, 5385–5398 (1986).
Li, Y., Guo, M. & Li, Y. Recent advances in plasticized PVC gels for soft actuators and devices: a review. J. Mater. Chem. C. 7, 12991–13009 (2019).
Wang, Z., et al. 3D printing of electrically responsive PVC gel actuators. ACS Appl. Mater. Interfaces 13, 24164–24172 (2021).
Hirai, T., et al. Electrically active artificial pupil showing amoeba-like pseudopodial deformation. Adv. Mater. 21, 2886–2888 (2009).
Ge, D. et al. A robust smart window: reversibly switching from high transparency to angle-independent structural color display. Adv. Mater. 27, 2489–2495 (2015).
Ke, Y. et al. Smart windows: electro-, thermo-, mechano-, photochromics, and beyond. Adv. Energy Mater. 9, 1902066 (2019).
Li, Y., Maeda, Y. & Hashimoto, M. Lightweight, soft variable stiffness gel spats for walking assistance. Int. J. Adv. Robot. 12, 175 (2015).
Kato, Y. et al. Sheet-type Braille displays by integrating organic field-effect transistors and polymeric actuators. IEEE Trans. Electron. Device. 54, 202–209 (2007).
Kameda, T., Ono, M., Grause, G., Mizoguchi, T. & Yoshioka, T. Chemical modification of poly(vinyl chloride) by nucleophilic substitution. Polym. Degrad. 94, 107–112 (2009).
Liu, R., et al. Assessing the influence of various imidazolium groups on the properties of poly(vinyl chloride) based high temperature proton exchange membranes. Eur. Polym. J. 137, 109948 (2020).
Choi, J. K., Kim, Y. W., Koh, J. H. & Kim, J. H. Proton conducting membranes based on poly(vinyl chloride) graft copolymer electrolytes. Adv. Polym. Tech. 19, 915–921 (2008).
Allan, J. T., Prest, L. E. & Easton, E. B. The sulfonation of polyvinyl chloride: synthesis and characterization for proton conducting membrane applications. J. Membr. Sci. 489, 175–182 (2015).
Nayak, V., Jyothi, M., Balakrishna, R. G., Padaki, M. & Isloor, A. M. Synthesis and characterization of novel sulfanilic acid–polyvinyl chloride–polysulfone blend membranes for metal ion rejection. RSC Adv. 6, 25492–25502 (2016).
Kim, J., Lee, J., Jo, C. & Kang, C. Development of low cost carbon fibers based on chlorinated polyvinyl chloride (CPVC) for automotive applications. Mater. Des. 204, 109682 (2021).
Forsyth, D. & Jay, B. Organotin leachates in drinking water from chlorinated poly(vinyl chloride) (CPVC) pipe. Appl. Organomet. Chem. 11, 551–558 (1997).
Marino, A. et al. ZSM-5 zeolites performance assessment in catalytic pyrolysis of PVC-containing real WEEE plastic wastes. Catal. Today. 390, 210–220 (2022).
Al-Harahsheh, M., Al-Nu’airat, J., Al-Otoom, A., Al-Jabali, H. & Al-Zoubi, M. Treatments of electric arc furnace dust and halogenated plastic wastes: a review. J. Environ. Chem. Eng. 7, 102856 (2019).
Muir, D., Stern, G. & Tomy, G. in Anthropogenic Compounds Part K. The Handbook of Environmental Chemistry Vol. 3K (eds Hutzinger, O. & Paasivirta, J.) 203–236 (Springer, 2000).
Morrison, R. D. & Murphy, B. L. Chlorinated Solvents: A Forensic Evaluation (RSC, 2015).
Mishra, S., Roy, M. & Mohanty, K. Microalgal bioenergy production under zero-waste biorefinery approach: recent advances and future perspectives. Bioresour. Technol. 292, 122008 (2019).
Brundage, M. P., Lechevalier, D. & Morris, K. C. Toward standards-based generation of reusable life cycle inventory data models for manufacturing processes. J. Manuf. Sci. Eng 141, 4041947 (2018).
Verma, R., Vinoda, K., Papireddy, M. & Gowda, A. Toxic pollutants from plastic waste — a review. Procedia Environ. Sci. 35, 701–708 (2016).
European Commission. The Behaviour of PVC in Landfill (Argus, 2021).
Martín, A. J., Mondelli, C., Jaydev, S. D. & Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 7, 1487–1533 (2021).
Everard, M. Twenty years of the polyvinyl chloride sustainability challenges. J. Vinyl Addit. Technol. 26, 390–402 (2020).
Yu, H. et al. Co-pyrolysis of biomass and polyvinyl chloride under microwave irradiation: distribution of chlorine. Sci. Total. Environ. 806, 150903 (2022).
Al-Harahsheh, M., Kingman, S., Al-Makhadmah, L. & Hamilton, I. E. Microwave treatment of electric arc furnace dust with PVC: dielectric characterization and pyrolysis-leaching. J. Hazard. Mater. 274, 87–97 (2014).
Zhao, S., Wang, C., Bai, B., Jin, H. & Wei, W. Study on the polystyrene plastic degradation in supercritical water/CO2 mixed environment and carbon fixation of polystyrene plastic in CO2 environment. J. Hazard. Mater. 421, 126763 (2022).
Xu, B., Zhao, X., Chen, H., Su, S. & Yu, H. Effects of carbonization conditions on structure and gas adsorption of carbon membranes derived from polyvinyl chloride. Chem. Sel. 5, 957–961 (2020).
Zhongming, Z., Linong, L., Xiaona, Y., Wangqiang, Z. & Wei, L. The New Plastics Economy Global Commitment 2020 Progress Report (UN Environment Programme, 2020).
Sato, Y., Kato, K., Takeshita, Y., Takahashi, K. & Nishi, S. Decomposition of ployvinylchloride using supercritical water. J. Appl. Phys. 37, 6270 (1998).
Du, J. et al. Environmental distribution, transport and ecotoxicity of microplastics: a review. J. Appl. Toxicol. 41, 52–64 (2021).
Dong, J. et al. Feasibility evaluation of the terminated waste energy in situ conversion strategy toward carbon neutralization in metallurgical processes. ACS Sustain. Chem. Eng. 9, 14079–14089 (2021).
Fantozzi, F., D’Alessandro, B. & Bidini, G. IPRP (integrated-pyrolysis regenerated plant): gas turbine and externally heated rotary-kiln pyrolysis as a biomass and waste energy conversion system. Influence of thermodynamic parameters. J. Power Energy 217, 519–527 (2003).
Genuino, H. C., Ruiz, M. P., Heeres, H. J. & Kersten, S. R. Pyrolysis of mixed plastic waste (DKR-350): effect of washing pre-treatment and fate of chlorine. Fuel Process. Technol. 233, 107304 (2022).
European Commission. The Usage of PVC (Polyvinyl Chloride) in the Context of a Non-Toxic Environment (Ramboll, 2020).
Acknowledgements
F.H. acknowledges support from the Key Research Program of Frontier Science, Chinese Academy of Sciences (Grant No. QYZDJ-SSW-JSC013). T.Z. acknowledges support from the Department of Engineering Science and Mechanics, the Materials Research Institute and the Huck Institutes of the Life Sciences at Pennsylvania State University. T.L. and S.X. acknowledge support from the National Natural Science Foundation (Grant No. 22205254 and 51922103). T.L. also acknowledges the support from the National Key Research and Development Program of China (Grant No. 2019YFA0210600) and Shanghai Pilot Program for Basic Research, Shanghai Jiao Tong University. The authors thank Modor Intelligence, McKinsey & Company and the PVC Association for providing global polyvinyl chloride (PVC) market and recovery data. Furthermore, the authors thank the reviewers for their time and efforts in improving this work.
Author information
Authors and Affiliations
Contributions
Introduction (S.X., Z.H., K.Y., P.Q., T.Z. and F.H.); Experimentation (S.X., Z.H., W.Z., T.Z. and F.H.); Results (S.X., T.Z. and F.H.); Applications (S.X., T.Z. and F.H.); Reproducibility and data deposition (S.X., T.L., T.Z. and F.H.); Limitations and optimizations (S.X., Z.H., K.Y., P.Q., W.Z., T.L., T.Z. and F.H.); Outlook (S.X., K.Y., P.Q., T.Z. and F.H.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Megan Fieser, Nancy Bush, Zach Wood and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Bio-accumulative toxicity
-
Toxins that persist and accumulate in the fatty tissues of animals and humans over time.
- Circular plastic recycling
-
Maintaining the economic value of plastics in a closed-loop system rather than using them just once and then disposing of them.
- Dehydrochlorination
-
An elimination process in which HCl is removed to form olefins.
- Hydrodechlorination
-
A substitution process in which chlorine is replaced by hydrogen with the help of electron donors such as a noble metal, metal oxides and silylium.
- Pyro-gasification
-
Pyro-gasification of plastic waste at 500–700 °C is based on the same natural waste fermentation as anaerobic digestion, with a small amount of oxygen introduced to enable the thermochemical process while preventing combustion.
- Solvent processing
-
Processing that generally involves using solvents to physically dissolve reactants or catalysts, or to serve as a reactive compound to produce novel mixtures or new chemicals.
- Supercritical H2O pyrolysis
-
A process in which plastic waste is heated in a pressurized environment of supercritical water, resulting in the breakdown of the plastic into smaller molecules.
- Syndiotactic sequences
-
Sequences of monomers that are arranged in a specific order and have a regular arrangement of substituents, resulting in a highly crystalline polymer structure.
- Thermosetting polymers
-
Polymers that form a permanent bond and become rigid when heated.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, S., Han, Z., Yuan, K. et al. Upcycling chlorinated waste plastics. Nat Rev Methods Primers 3, 44 (2023). https://doi.org/10.1038/s43586-023-00227-w
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00227-w
This article is cited by
-
Upcycling chlorinated trash into synthetic organic treasure
Nature Chemistry (2024)