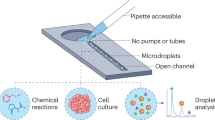
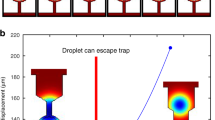
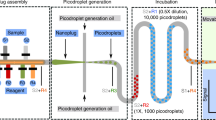
Abstract
Droplet-based microfluidic systems generate, manipulate and control sub-microlitre droplets enclosed within an immiscible carrier fluid. Owing to a number of remarkable features, such as the ability to precisely control the chemical and biological payload of each droplet and to produce thousands of droplets per second, this technology is transforming how chemists and biologists perform high-throughput or massively parallel experiments. In this Primer, we initially introduce and describe the basic features of droplet-based microfluidic systems and key issues that should be considered when developing new chemical and biological workflows. We provide a critical evaluation of how droplet-based microfluidic systems should be manufactured and the importance of integrating appropriate detection technologies to probe the small analytical volumes that are representatives of the technology set. We then discuss issues related to data collection and management, providing guidelines on how large data sets should be processed and manipulated. Furthermore, we showcase some of the most successful and important applications of droplet-based systems in the biological and chemical sciences and consider issues that currently hinder progress in both technology development and application. Finally, we provide some opinion on future directions for the technology set and where its greatest impact will be felt in the coming years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ding, Y., Howes, P. D. & deMello, A. J. Recent advances in droplet microfluidics. Anal. Chem. 92, 132–149 (2020).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Chiu, D. T. et al. Small but perfectly formed? Successes, challenges, and opportunities for microfluidics in the chemical and biological sciences. Chem 2, 201–223 (2017).
Squires, T. M. & Quake, S. R. Microfluidics: fluid physics at the nanoliter scale. Rev. Mod. Phys. 77, 977–1026 (2005).
Song, H., Chen, D. L. & Ismagilov, R. F. Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed. 45, 7336–7356 (2006).
Kawakatsu, T., Kikuchi, Y. & Nakajima, M. Regular-sized cell creation in microchannel emulsification by visual microprocessing method. J. Am. Oil Chem. Soc. 74, 317–321 (1997).
Thorsen, T., Roberts, R. W., Arnold, F. H. & Quake, S. R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 86, 4163–4166 (2001).
Umbanhowar, P. B., Prasad, V. & Weitz, D. A. Monodisperse emulsion generation via drop break off in a coflowing stream. Langmuir 16, 347–351 (2000).
Shang, L., Cheng, Y. & Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 117, 7964–8040 (2017).
Matuła, K., Rivello, F. & Huck, W. T. S. Single-cell analysis using droplet microfluidics. Adv. Biosyst. 4, 1900188 (2020).
Lignos, I. et al. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: fast parametric space mapping. Nano Lett. 16, 1869–1877 (2016).
Obexer, R., Pott, M., Zeymer, C., Griffiths, A. D. & Hilvert, D. Efficient laboratory evolution of computationally designed enzymes with low starting activities using fluorescence-activated droplet sorting. Protein Eng. Des. Sel. 29, 355–366 (2016).
Kleine-Brüggeney, H. et al. Long-term perfusion culture of monoclonal embryonic stem cells in 3D hydrogel beads for continuous optical analysis of differentiation. Small 15, 1804576 (2019).
Abolhasani, M. et al. Oscillatory microprocessor for growth and in situ characterization of semiconductor nanocrystals. Chem. Mater. 27, 6131–6138 (2015).
Xia, Y. & Whitesides, G. M. Soft lithography. Angew. Chem. Int. Ed. 37, 550–575 (1998).
Trantidou, T., Elani, Y., Parsons, E. & Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 3, 16091 (2017).
Chen, F. et al. Chemical transfection of cells in picoliter aqueous droplets in fluorocarbon oil. Anal. Chem. 83, 8816–8820 (2011).
Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 12, 422–433 (2012).
Holtze, C. et al. Biocompatible surfactants for water-in-fluorocarbon emulsions. Lab Chip 8, 1632–1639 (2008).
Lee, J. N., Park, C. & Whitesides, G. M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 75, 6544–6554 (2003).
Toepke, M. W. & Beebe, D. J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 6, 1484 (2006).
Kim, J., deMello, A. J., Chang, S.-I., Hong, J. & O’Hare, D. Thermoset polyester droplet-based microfluidic devices for high frequency generation. Lab Chip 11, 4108–4112 (2011).
Wu, N. et al. A PMMA microfluidic droplet platform for in vitro protein expression using crude E. coli S30 extract. Lab Chip 9, 3391 (2009).
Su, S. et al. One-step bonding and hydrophobic surface modification method for rapid fabrication of polycarbonate-based droplet microfluidic chips. Sens. Actuators B Chem. 282, 60–68 (2019).
Li, H., Fan, Y., Kodzius, R. & Foulds, I. G. Fabrication of polystyrene microfluidic devices using a pulsed CO2 laser system. Microsyst. Technol. 18, 373–379 (2012).
Aghvami, S. A. et al. Rapid prototyping of cyclic olefin copolymer (COC) microfluidic devices. Sens. Actuators B Chem. 247, 940–949 (2017).
Ren, K., Dai, W., Zhou, J., Su, J. & Wu, H. Whole-Teflon microfluidic chips. Proc. Natl Acad. Sci. USA 108, 8162–8166 (2011).
Meng, Z.-J. et al. Plug-n-play microfluidic systems from flexible assembly of glass-based flow-control modules. Lab Chip 15, 1869–1878 (2015).
Zhu, P. & Wang, L. Passive and active droplet generation with microfluidics: a review. Lab Chip 17, 34–75 (2016).
Anna, S. L., Bontoux, N. & Stone, H. A. Formation of dispersions using ‘flow focusing’ in microchannels. Appl. Phys. Lett. 82, 364–366 (2003).
Tice, J. D., Song, H., Lyon, A. D. & Ismagilov, R. F. Formation of droplets and mixing in multiphase microfluidics at low values of the Reynolds and the capillary numbers. Langmuir 19, 9127–9133 (2003).
Dangla, R., Kayi, S. C. & Baroud, C. N. Droplet microfluidics driven by gradients of confinement. Proc. Natl Acad. Sci. USA 110, 853–858 (2013).
Utada, A. S. et al. Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 (2005).
Chu, L.-Y., Utada, A. S., Shah, R. K., Kim, J.-W. & Weitz, D. A. Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed. 46, 8970–8974 (2007).
Huebner, A. et al. Quantitative detection of protein expression in single cells using droplet microfluidics. Chem. Commun. https://doi.org/10.1039/b618570c (2007).
Kiss, M. M. et al. High-throughput quantitative polymerase chain reaction in picoliter droplets. Anal. Chem. 80, 8975–8981 (2008).
Shi, W., Qin, J., Ye, N. & Lin, B. Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip 8, 1432–1435 (2008).
Delley, C. L. & Abate, A. R. Microfluidic particle zipper enables controlled loading of droplets with distinct particle types. Lab Chip 20, 2465–2472 (2020).
Song, H. & Ismagilov, R. F. Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc. 125, 14613–14619 (2003).
Bremond, N., Thiam, A. R. & Bibette, J. Decompressing emulsion droplets favors coalescence. Phys. Rev. Lett. 100, 024501 (2008).
Niu, X., Gulati, S., Edel, J. B. & deMello, A. J. Pillar-induced droplet merging in microfluidic circuits. Lab Chip 8, 1837–1841 (2008).
Sesen, M., Alan, T. & Neild, A. Microfluidic on-demand droplet merging using surface acoustic waves. Lab Chip 14, 3325–3333 (2014).
Niu, X., Gielen, F., deMello, A. J. & Edel, J. B. Electro-coalescence of digitally controlled droplets. Anal. Chem. 81, 7321–7325 (2009).
Abate, A. R., Hung, T., Mary, P., Agresti, J. J. & Weitz, D. A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl Acad. Sci. USA 107, 19163–19166 (2010).
Song, H., Tice, J. D. & Ismagilov, R. F. A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed. 42, 768–772 (2003).
Arun Sankar, E. M., Shahab, M. & Rengaswamy, R. Spacing optimization for active droplet sorting in microfluidic networks using genetic algorithm. Ind. Eng. Chem. Res. 60, 1699–1708 (2021).
Hess, D. et al. Exploring mechanism of enzyme catalysis by on-chip transient kinetics coupled with global data analysis and molecular modeling. Chem 7, 1066–1079 (2021).
Baret, J.-C. et al. Fluorescence-activated droplet sorting (FADS): efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 9, 1850–1858 (2009).
Schmid, L., Weitz, D. A. & Franke, T. Sorting drops and cells with acoustics: acoustic microfluidic fluorescence-activated cell sorter. Lab Chip 14, 3710–3718 (2014).
Abate, A. R., Agresti, J. J. & Weitz, D. A. Microfluidic sorting with high-speed single-layer membrane valves. Appl. Phys. Lett. 96, 203509 (2010).
Niu, X., Gielen, F., Edel, J. B. & deMello, A. J. A microdroplet dilutor for high-throughput screening. Nat. Chem. 3, 437–442 (2011).
Frenz, L., Blouwolff, J., Griffiths, A. D. & Baret, J.-C. Microfluidic production of droplet pairs. Langmuir 24, 12073–12076 (2008).
Zheng, B., Tice, J. D. & Ismagilov, R. F. Formation of droplets of alternating composition in microfluidic channels and applications to indexing of concentrations in droplet-based assays. Anal. Chem. 76, 4977–4982 (2004).
Huebner, A. et al. Static microdroplet arrays: a microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 9, 692–698 (2009).
Frenz, L., Blank, K., Brouzes, E. & Griffiths, A. D. Reliable microfluidic on-chip incubation of droplets in delay-lines. Lab Chip 9, 1344–1348 (2009).
Bringer, M. R., Gerdts, C. J., Song, H., Tice, J. D. & Ismagilov, R. F. Microfluidic systems for chemical kinetics that rely on chaotic mixing in droplets. Philos. Transact. A Math. Phys. Eng. Sci. 362, 1087–1104 (2004).
Cole, R. H., Lange, N., de, Gartner, Z. J. & Abate, A. R. Compact and modular multicolour fluorescence detector for droplet microfluidics. Lab Chip 15, 2754–2758 (2015).
Hess, D., Rane, A., deMello, A. J. & Stavrakis, S. High-throughput, quantitative enzyme kinetic analysis in microdroplets using stroboscopic epifluorescence imaging. Anal. Chem. 87, 4965–4972 (2015).
Benninger, R. K. P. et al. Fluorescence-lifetime imaging of DNA-dye interactions within continuous-flow microfluidic systems. Angew. Chem. Int. Ed. 46, 2228–2231 (2007).
Probst, J., Howes, P., Arosio, P., Stavrakis, S. & deMello, A. Broad-band spectrum, high-sensitivity absorbance spectroscopy in picoliter volumes. Anal. Chem. 93, 7673–7681 (2021).
Probst, J. et al. In situ X-ray absorption spectroscopy and droplet-based microfluidics: an analysis of calcium carbonate precipitation. ACS Meas. Sci. Au 1, 27–34 (2021).
Cristobal, G. et al. On-line laser Raman spectroscopic probing of droplets engineered in microfluidic devices. Lab Chip 6, 1140–1146 (2006).
Strehle, K. R. et al. A reproducible surface-enhanced Raman spectroscopy approach. Online SERS measurements in a segmented microfluidic system. Anal. Chem. 79, 1542–1547 (2007).
Chan, K. L. A. & Kazarian, S. G. FT-IR spectroscopic imaging of reactions in multiphase flow in microfluidic channels. Anal. Chem. 84, 4052–4056 (2012).
Maceiczyk, R. M., Hess, D., Chiu, F. W. Y., Stavrakis, S. & deMello, A. J. Differential detection photothermal spectroscopy: towards ultra-fast and sensitive label-free detection in picoliter & femtoliter droplets. Lab Chip 17, 3654–3663 (2017).
Cecchini, M. P. et al. Ultrafast surface enhanced resonance Raman scattering detection in droplet-based microfluidic systems. Anal. Chem. 83, 3076–3081 (2011).
Gasilova, N., Yu, Q., Qiao, L. & Girault, H. H. On-chip spyhole mass spectrometry for droplet-based microfluidics. Angew. Chem. Int. Ed. 53, 4408–4412 (2014).
Steyer, D. J. & Kennedy, R. T. High-throughput nanoelectrospray ionization-mass spectrometry analysis of microfluidic droplet samples. Anal. Chem. 91, 6645–6651 (2019).
Kempa, E. E. et al. Coupling droplet microfluidics with mass spectrometry for ultrahigh-throughput analysis of complex mixtures up to and above 30 Hz. Anal. Chem. 92, 12605–12612 (2020).
Petit-Pierre, G. et al. In vivo neurochemical measurements in cerebral tissues using a droplet-based monitoring system. Nat. Commun. 8, 1239 (2017).
Bell, S. E. et al. Droplet microfluidics with MALDI-MS detection: the effects of oil phases in GABA analysis. ACS Meas. Sci. Au 1, 147–156 (2021).
Clark, I. C. & Abate, A. R. Finding a helix in a haystack: nucleic acid cytometry with droplet microfluidics. Lab Chip 17, 2032–2045 (2017).
Stucki, A., Vallapurackal, J., Ward, T. R. & Dittrich, P. S. Droplet microfluidics and directed evolution of enzymes: an intertwined journey. Angew. Chem. Int. Ed. 60, 24368–24387 (2021).
Kim, J.-W. et al. Recent advances in the microfluidic production of functional microcapsules by multiple-emulsion templating. Lab Chip 22, 2259–2291 (2022).
Chen, M., Bolognesi, G. & Vladisavljević, G. T. Crosslinking strategies for the microfluidic production of microgels. Molecules 26, 3752 (2021).
Seiffert, S. Microgel capsules tailored by droplet-based microfluidics. ChemPhysChem 14, 295–304 (2013).
Zhu, Z. & Yang, C. J. Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 50, 22–31 (2017).
Wu, J., Yadavali, S., Lee, D. & Issadore, D. A. Scaling up the throughput of microfluidic droplet-based materials synthesis: a review of recent progress and outlook. Appl. Phys. Rev. 8, 031304 (2021).
Håti, A. G. et al. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab Chip 16, 3718–3727 (2016).
Ahn, K., Agresti, J., Chong, H., Marquez, M. & Weitz, D. A. Electrocoalescence of drops synchronized by size-dependent flow in microfluidic channels. Appl. Phys. Lett. 88, 264105 (2006).
Califano, D. et al. Enzyme-functionalized cellulose beads as a promising antimicrobial material. Biomacromolecules 22, 754–762 (2021).
Hsu, M. N. et al. Smart hydrogel microfluidics for single-cell multiplexed secretomic analysis with high sensitivity. Small Weinh. Bergstr. Ger. 14, e1802918 (2018).
Klein, A. M. et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015).
Weisgerber, D. W., Hatori, M., Li, X. & Abate, A. R. Polyhedral particles with controlled concavity by indentation templating. Anal. Chem. 94, 7475–7482 (2022).
Abbaspourrad, A., Carroll, N. J., Kim, S.-H. & Weitz, D. A. Surface functionalized hydrophobic porous particles toward water treatment application. Adv. Mater. 25, 3215–3221 (2013).
Song, Y., Sauret, A. & Cheung Shum, H. All-aqueous multiphase microfluidics. Biomicrofluidics 7, 61301 (2013).
Vijayakumar, K., Gulati, S., deMello, A. J. & Edel, J. B. Rapid cell extraction in aqueous two-phase microdroplet systems. Chem. Sci. 1, 447–452 (2010).
Kim, B.-Y., Hong, L.-Y., Chung, Y.-M., Kim, D.-P. & Lee, C.-S. Solvent-resistant PDMS microfluidic devices with hybrid inorganic/organic polymer coatings. Adv. Funct. Mater. 19, 3796–3803 (2009).
Rolland, J. P., Van Dam, R. M., Schorzman, D. A., Quake, S. R. & DeSimone, J. M. Solvent-resistant photocurable liquid fluoropolymers for microfluidic device fabrication [corrected]. J. Am. Chem. Soc. 126, 2322–2323 (2004).
Benson, B. R., Stone, H. A. & Prud’homme, R. K. An ‘off-the-shelf’ capillary microfluidic device that enables tuning of the droplet breakup regime at constant flow rates. Lab Chip 13, 4507–4511 (2013).
Vladisavljević, G. T., Al Nuumani, R. & Nabavi, S. A. Microfluidic production of multiple emulsions. Micromachines 8, 75 (2017).
Vega-Vásquez, P., Mosier, N. S. & Irudayaraj, J. Nanoscale drug delivery systems: from medicine to agriculture. Front. Bioeng. Biotechnol. 8, 79 (2020).
van der Kooij, R. S., Steendam, R., Frijlink, H. W. & Hinrichs, W. L. J. An overview of the production methods for core-shell microspheres for parenteral controlled drug delivery. Eur. J. Pharm. Biopharm. 170, 24–42 (2022).
Zhu, P. & Wang, L. in Microfluidics-Enabled Soft Manufacture (eds Zhu, P. & Wang, L.) 89–104 (Springer International Publishing, 2022).
Sciambi, A. & Abate, A. R. Adding reagent to droplets with controlled rupture of encapsulated double emulsions. Biomicrofluidics 7, 44112 (2013).
Jia, Y. et al. Sequential coalescence enabled two-step microreactions in triple-core double-emulsion droplets triggered by an electric field. Small 13, 1702188 (2017).
Chen, C.-H., Shah, R. K., Abate, A. R. & Weitz, D. A. Janus particles templated from double emulsion droplets generated using microfluidics. Langmuir 25, 4320–4323 (2009).
Seo, M., Paquet, C., Nie, Z., Xu, S. & Kumacheva, E. Microfluidic consecutive flow-focusing droplet generators. Soft Matter 3, 986–992 (2007).
Abate, A. R., Lee, D., Do, T., Holtze, C. & Weitz, D. A. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip 8, 516–518 (2008).
Abate, A. R. et al. Photoreactive coating for high-contrast spatial patterning of microfluidic device wettability. Lab Chip 8, 2157–2160 (2008).
Abate, A. R., Thiele, J., Weinhart, M. & Weitz, D. A. Patterning microfluidic device wettability using flow confinement. Lab Chip 10, 1774–1776 (2010).
Kim, S. C., Sukovich, D. J. & Abate, A. R. Patterning microfluidic device wettability with spatially-controlled plasma oxidation. Lab Chip 15, 3163–3169 (2015).
Duncanson, W. J. et al. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip 12, 2135–2145 (2012).
Tran, T. M., Cater, S. & Abate, A. R. Coaxial flow focusing in poly(dimethylsiloxane) microfluidic devices. Biomicrofluidics 8, 016502 (2014).
Rodriguez-Trujillo, R., Kim-Im, Y.-H. & Hernandez-Machado, A. Controlling shapes in a coaxial flow focusing microfluidic device: experiments and theory. Micromachines 11, E85 (2020).
Cole, R. H., Tran, T. M. & Abate, A. R. Double emulsion generation using a polydimethylsiloxane (PDMS) co-axial flow focus device. J. Vis. Exp. (2015).
Mazutis, L. et al. Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal. Chem. 81, 4813–4821 (2009).
Hadikhani, P., Borhani, N., H Hashemi, S. M. & Psaltis, D. Learning from droplet flows in microfluidic channels using deep neural networks. Sci. Rep. 9, 8114 (2019).
Paegel, B. M. & Joyce, G. F. Microfluidic compartmentalized directed evolution. Chem. Biol. 17, 717–724 (2010).
Fruncillo, S. et al. Lithographic processes for the scalable fabrication of micro- and nanostructures for biochips and biosensors. ACS Sens. 6, 2002–2024 (2021).
Polizzi, K. M. et al. Pooling for improved screening of combinatorial libraries for directed evolution. Biotechnol. Prog. 22, 961–967 (2006).
Herzenberg, L. A. et al. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin. Chem. 48, 1819–1827 (2002).
Wunder, F., Kalthof, B., Muller, T. & Huser, J. Functional cell-based assays in microliter volumes for ultra-high throughput screening. Comb. Chem. High. Throughput Screen. 11, 495–504 (2008).
Kaminski, T. S., Scheler, O. & Garstecki, P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip 16, 2168–2187 (2016).
Tawfik, D. S. & Griffiths, A. D. Man-made cell-like compartments for molecular evolution. Nat. Biotechnol. 16, 652–656 (1998).
Sciambi, A. & Abate, A. R. Accurate microfluidic sorting of droplets at 30 kHz. Lab Chip 15, 47–51 (2015).
Cobb, R. E., Chao, R. & Zhao, H. Directed evolution: past, present and future. AIChE 59, 1432–1440 (2013).
van Tatenhove-Pel, R. J. et al. Microdroplet screening and selection for improved microbial production of extracellular compounds. Curr. Opin. Biotechnol. 61, 72–81 (2020).
Yu, Z. et al. Droplet-based microfluidic screening and sorting of microalgal populations for strain engineering applications. Algal Res. 56, 102293 (2021).
Madrigal, J. L. et al. Characterizing cell interactions at scale with made-to-order droplet ensembles (MODEs). Proc. Natl Acad. Sci. USA 119, e2110867119 (2022).
Colin, P.-Y. et al. Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics. Nat. Commun. 6, 10008 (2015).
Najah, M. et al. Droplet-based microfluidics platform for ultra-high-throughput bioprospecting of cellulolytic microorganisms. Chem. Biol. 21, 1722–1732 (2014).
Shembekar, N., Chaipan, C., Utharala, R. & Merten, C. A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 16, 1314–1331 (2016).
Terekhov, S. S. et al. Ultrahigh-throughput functional profiling of microbiota communities. Proc. Natl Acad. Sci. USA 115, 9551–9556 (2018).
Kulesa, A., Kehe, J., Hurtado, J. E., Tawde, P. & Blainey, P. C. Combinatorial drug discovery in nanoliter droplets. Proc. Natl Acad. Sci. USA 115, 6685–6690 (2018).
Payne, E. M., Holland-Moritz, D. A., Sun, S. & Kennedy, R. T. High-throughput screening by droplet microfluidics: perspective into key challenges and future prospects. Lab Chip 20, 2247–2262 (2020).
Periyannan Rajeswari, P. K., Joensson, H. N. & Andersson-Svahn, H. Droplet size influences division of mammalian cell factories in droplet microfluidic cultivation. Electrophoresis 38, 305–310 (2017).
Gruner, P. et al. Controlling molecular transport in minimal emulsions. Nat. Commun. 7, 10392 (2016).
Ostafe, R., Prodanovic, R., Lloyd Ung, W., Weitz, D. A. & Fischer, R. A high-throughput cellulase screening system based on droplet microfluidics. Biomicrofluidics 8, 041102 (2014).
Abatemarco, J. et al. RNA-aptamers-in-droplets (RAPID) high-throughput screening for secretory phenotypes. Nat. Commun. 8, 332 (2017).
Yanakieva, D. et al. FACS-based functional protein screening via microfluidic co-encapsulation of yeast secretor and mammalian reporter cells. Sci. Rep. 10, 10182 (2020).
Holland-Moritz, D. A. et al. Mass activated droplet sorting (MADS) enables high-throughput screening of enzymatic reactions at nanoliter scale. Angew. Chem. Int. Ed. 59, 4470–4477 (2020).
Xu, L. et al. Mapping enzyme catalysis with metabolic biosensing. Nat. Commun. 12, 6803 (2021).
Cole, R. H. et al. Printed droplet microfluidics for on demand dispensing of picoliter droplets and cells. Proc. Natl Acad. Sci. USA 114, 8728–8733 (2017).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 9, 171–181 (2014).
DeLaughter, D. M. The use of the fluidigm C1 for RNA expression analyses of single cells. Curr. Protoc. Mol. Biol. 122, 55 (2018).
Zhou, W. et al. Microfluidics applications for high-throughput single cell sequencing. J. Nanobiotechnol. 19, 312 (2021).
Liu, L. et al. Methods and platforms for analysis of nucleic acids from single-cell based on microfluidics. Microfluid. Nanofluidics 25, 87 (2021).
Agresti, J. J. et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl Acad. Sci. USA 107, 4004–4009 (2010).
Lan, F., Demaree, B., Ahmed, N. & Abate, A. R. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 35, 640–646 (2017).
Denisenko, E. et al. Systematic assessment of tissue dissociation and storage biases in single-cell and single-nucleus RNA-seq workflows. Genome Biol. 21, 130 (2020).
Nguyen, Q. H., Pervolarakis, N., Nee, K. & Kessenbrock, K. Experimental considerations for single-cell RNA sequencing approaches. Front. Cell Dev. Biol. 6, 108 (2018).
Zielinski, J. M., Luke, J. J., Guglietta, S. & Krieg, C. High throughput multi-omics approaches for clinical trial evaluation and drug discovery. Front. Immunol. 12, 590742 (2021).
Jing, W. & Han, H.-S. Droplet microfluidics for high-resolution virology. Anal. Chem. 94, 8085–8100 (2022).
Tabula Sapiens Consortium*. The Tabula sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Tabula Muris Consortium. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Li, H. et al. Fly cell atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022).
Packer, J. S. et al. A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 365, eaax1971 (2019).
Jiang, M. et al. Characterization of the zebrafish cell landscape at single-cell resolution. Front. Cell Dev. Biol. 9, 743421 (2021).
Tsiatis, A. C. et al. Comparison of sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations. J. Mol. Diagn. 12, 425–432 (2010).
Hindson, B. J. et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 83, 8604–8610 (2011).
Men, Y. et al. Digital polymerase chain reaction in an array of femtoliter polydimethylsiloxane microreactors. Anal. Chem. 84, 4262–4266 (2012).
Alcaide, M. et al. Evaluating the quantity, quality and size distribution of cell-free DNA by multiplex droplet digital PCR. Sci. Rep. 10, 12564 (2020).
de Kock, R., Deiman, B., Kraaijvanger, R. & Scharnhorst, V. Optimized (pre) analytical conditions and workflow for droplet digital PCR analysis of cell-free DNA from patients with suspected lung carcinoma. J. Mol. Diagn. 21, 895–902 (2019).
Zeng, Y.-F. et al. Development of a droplet digital PCR method for detection of Streptococcus agalactiae. BMC Microbiol. 20, 179 (2020).
Vasudevan, H. N. et al. Digital droplet PCR accurately quantifies SARS-CoV-2 viral load from crude lysate without nucleic acid purification. Sci. Rep. 11, 780 (2021).
Chang, M. Y. et al. One-step noninvasive prenatal testing (NIPT) for autosomal recessive homozygous point mutations using digital PCR. Sci. Rep. 8, 2877 (2018).
Clausell-Tormos, J. et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 15, 427–437 (2008).
Kintses, B. et al. Picoliter cell lysate assays in microfluidic droplet compartments for directed enzyme evolution. Chem. Biol. 19, 1001–1009 (2012).
Obexer, R. et al. Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase. Nat. Chem. 9, 50–56 (2017).
Debon, A. et al. Ultrahigh-throughput screening enables efficient single-round oxidase remodelling. Nat. Catal. 2, 740–747 (2019).
Shembekar, N., Hu, H., Eustace, D. & Merten, C. A. Single-cell droplet microfluidic screening for antibodies specifically binding to target cells. Cell Rep. 22, 2206–2215 (2018).
Gérard, A. et al. High-throughput single-cell activity-based screening and sequencing of antibodies using droplet microfluidics. Nat. Biotechnol. 38, 715–721 (2020).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Gielen, F. et al. Ultrahigh-throughput-directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proc. Natl Acad. Sci. 113, E7383–E7389 (2016).
Boitard, L. et al. Monitoring single-cell bioenergetics via the coarsening of emulsion droplets. Proc. Natl Acad. Sci. USA 109, 7181–7186 (2012).
Eyer, K. et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat. Biotechnol. 35, 977–982 (2017).
Bounab, Y. et al. Dynamic single-cell phenotyping of immune cells using the microfluidic platform DropMap. Nat. Protoc. 15, 2920–2955 (2020).
Bucheli, O. T. M., Sigvaldadóttir, I. & Eyer, K. Measuring single-cell protein secretion in immunology: technologies, advances, and applications. Eur. J. Immunol. 51, 1334–1347 (2021).
Heo, M. et al. Deep phenotypic characterization of immunization-induced antibacterial IgG repertoires in mice using a single-antibody bioassay. Commun. Biol. 3, 614 (2020).
Llitjos, J.-F. et al. Assessing the functional heterogeneity of monocytes in human septic shock: a proof-of-concept microfluidic assay of TNFα secretion. Front. Immunol. 12, 686111 (2021).
Subedi, N. et al. An automated real-time microfluidic platform to probe single NK cell heterogeneity and cytotoxicity on-chip. Sci. Rep. 11, 17084 (2021).
Eyer, K. et al. The quantitative assessment of the secreted IgG repertoire after recall to evaluate the quality of immunizations. J. Immunol. 205, 1176–1184 (2020).
Kräutler, N. J. et al. Quantitative and qualitative analysis of humoral immunity reveals continued and personalized evolution in chronic viral infection. Cell Rep. 30, 997–1012 (2020).
Canales-Herrerias, P. et al. High-affinity autoreactive plasma cells disseminate through multiple organs in patients with immune thrombocytopenic purpura. J. Clin. Invest. 132, e153580 (2022).
Molari, M., Eyer, K., Baudry, J., Cocco, S. & Monasson, R. Quantitative modeling of the effect of antigen dosage on B-cell affinity distributions in maturating germinal centers. eLife 9, e55678 (2020).
Woronoff, G. et al. Metabolic cost of rapid adaptation of single yeast cells. Proc. Natl Acad. Sci. USA 117, 10660–10666 (2020).
Abbyad, P., Dangla, R., Alexandrou, A. & Baroud, C. N. Rails and anchors: guiding and trapping droplet microreactors in two dimensions. Lab Chip 11, 813–821 (2011).
Amselem, G., Guermonprez, C., Drogue, B., Michelin, S. & Baroud, C. N. Universal microfluidic platform for bioassays in anchored droplets. Lab Chip 16, 4200–4211 (2016).
Zilionis, R. et al. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 12, 44–73 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Kivioja, T. et al. Counting absolute numbers of molecules using unique molecular identifiers. Nat. Methods 9, 72–74 (2012).
Islam, S. et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods 11, 163–166 (2014).
Grosselin, K. et al. High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat. Genet. 51, 1060–1066 (2019).
Abate, A. R., Chen, C.-H., Agresti, J. J. & Weitz, D. A. Beating Poisson encapsulation statistics using close-packed ordering. Lab Chip 9, 2628–2631 (2009).
Goldstein, L. D. et al. Massively parallel single-cell B-cell receptor sequencing enables rapid discovery of diverse antigen-reactive antibodies. Commun. Biol. 2, 304 (2019).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Wu, T. D. et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 579, 274–278 (2020).
Tu, A. A. et al. TCR sequencing paired with massively parallel 3′ RNA-seq reveals clonotypic T cell signatures. Nat. Immunol. 20, 1692–1699 (2019).
Singh, M. et al. High-throughput targeted long-read single cell sequencing reveals the clonal and transcriptional landscape of lymphocytes. Nat. Commun. 10, 3120 (2019).
Lareau, C. A. et al. Droplet-based combinatorial indexing for massive-scale single-cell chromatin accessibility. Nat. Biotechnol. 37, 916–924 (2019).
Chen, S., Lake, B. B. & Zhang, K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat. Biotechnol. 37, 1452–1457 (2019).
De Rop, F. V. et al. Hydrop enables droplet-based single-cell ATAC-seq and single-cell RNA-seq using dissolvable hydrogel beads. eLife 11, e73971 (2022).
Rotem, A. et al. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nat. Biotechnol. 33, 1165–1172 (2015).
Stoeckius, M. et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017).
Debs, B. E., Utharala, R., Balyasnikova, I. V., Griffiths, A. D. & Merten, C. A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl Acad. Sci. USA 109, 11570–11575 (2012).
Wang, Y. et al. High-throughput functional screening for next-generation cancer immunotherapy using droplet-based microfluidics. Sci. Adv. 7, eabe3839 (2021).
Packer, M. S. & Liu, D. R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 16, 379–394 (2015).
Manteca, A. et al. Directed evolution in drops: molecular aspects and applications. ACS Synth. Biol. 10, 2772–2783 (2021).
Zeymer, C. & Hilvert, D. Directed evolution of protein catalysts. Annu. Rev. Biochem. https://doi.org/10.1146/annurev-biochem-062917-012034 (2018).
Neun, S., Zurek, P. J., Kaminski, T. S. & Hollfelder, F. in Methods in Enzymology (ed. Tawfik, D. S.) Ch. 13, 317–343 (Academic Press, 2020).
Weng, L. & Spoonamore, J. E. Droplet microfluidics-enabled high-throughput screening for protein engineering. Micromachines 10, 734 (2019).
Beneyton, T. et al. Droplet-based microfluidic high-throughput screening of heterologous enzymes secreted by the yeast Yarrowia lipolytica. Microb. Cell Factories 16, 18 (2017).
Beneyton, T. et al. High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci. Rep. 6, 27223 (2016).
Ryckelynck, M. et al. Using droplet-based microfluidics to improve the catalytic properties of RNA under multiple-turnover conditions. RNA 21, 458–469 (2015).
Bouhedda, F. et al. A dimerization-based fluorogenic dye-aptamer module for RNA imaging in live cells. Nat. Chem. Biol. 16, 69–76 (2020).
Trachman, R. J. et al. Structure and functional reselection of the Mango-III fluorogenic RNA aptamer. Nat. Chem. Biol. 15, 472–479 (2019).
Autour, A., Bouhedda, F., Cubi, R. & Ryckelynck, M. Optimization of fluorogenic RNA-based biosensors using droplet-based microfluidic ultrahigh-throughput screening. Methods 161, 46–53 (2019).
Autour, A. et al. Fluorogenic RNA Mango aptamers for imaging small non-coding RNAs in mammalian cells. Nat. Commun. 9, 656 (2018).
Autour, A., Westhof, E. & Ryckelynck, M. iSpinach: a fluorogenic RNA aptamer optimized for in vitro applications. Nucleic Acids Res. 44, 2491–2500 (2016).
Fischlechner, M. et al. Evolution of enzyme catalysts caged in biomimetic gel-shell beads. Nat. Chem. 6, 791–796 (2014).
Larsen, A. C. et al. A general strategy for expanding polymerase function by droplet microfluidics. Nat. Commun. 7, 11235 (2016).
Ma, F. et al. Efficient molecular evolution to generate enantioselective enzymes using a dual-channel microfluidic droplet screening platform. Nat. Commun. 9, 1030 (2018).
Prodanović, R. et al. A high-throughput screening system based on droplet microfluidics for glucose oxidase gene libraries. Molecules 25, 2418 (2020).
van Loo, B. et al. High-throughput, lysis-free screening for sulfatase activity using Escherichia coli autodisplay in microdroplets. ACS Synth. Biol. 8, 2690–2700 (2019).
Tomasi, R. F.-X., Sart, S., Champetier, T. & Baroud, C. N. Individual control and quantification of 3D spheroids in a high-density microfluidic droplet array. Cell Rep. 31, 107670 (2020).
Theberge, A. B. et al. Microfluidic platform for combinatorial synthesis in picolitre droplets. Lab Chip 12, 1320–1326 (2012).
Bannock, J. H. et al. Continuous synthesis of device-grade semiconducting polymers in droplet-based microreactors. Adv. Funct. Mater. 23, 2123–2129 (2013).
Kreutz, J. E. et al. Evolution of catalysts directed by genetic algorithms in a plug-based microfluidic device tested with oxidation of methane by oxygen. J. Am. Chem. Soc. 132, 3128–3132 (2010).
Faustini, M. et al. Microfluidic approach toward continuous and ultrafast synthesis of metal–organic framework crystals and hetero structures in confined microdroplets. J. Am. Chem. Soc. 135, 14619–14626 (2013).
Bawazer, L. A. et al. Combinatorial microfluidic droplet engineering for biomimetic material synthesis. Sci. Adv. 2, e1600567 (2016).
Nette, J., Howes, P. D. & deMello, A. J. Microfluidic synthesis of luminescent and plasmonic nanoparticles: fast, efficient, and data-rich. Adv. Mater. Technol. 5, 2000060 (2020).
Shestopalov, I., Tice, J. D. & Ismagilov, R. F. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip 4, 316–321 (2004).
Lignos, I. et al. Exploration of near-infrared-emissive colloidal multinary lead halide perovskite nanocrystals using an automated microfluidic platform. ACS Nano 12, 5504–5517 (2018).
Kuehne, A. J. C. & Weitz, D. A. Highly monodisperse conjugated polymer particles synthesized with drop-based microfluidics. Chem. Commun. 47, 12379 (2011).
Abolhasani, M., Oskooei, A., Klinkova, A., Kumacheva, E. & Günther, A. Shaken, and stirred: oscillatory segmented flow for controlled size-evolution of colloidal nanomaterials. Lab Chip 14, 2309–2318 (2014).
Li, S. et al. Precision tuning of rare-earth-doped upconversion nanoparticles via droplet-based microfluidic screening. J. Mater. Chem. C. 9, 925–933 (2021).
Fallah-Araghi, A. et al. Enhanced chemical synthesis at soft interfaces: a universal reaction-adsorption mechanism in microcompartments. Phys. Rev. Lett. 112, 028301 (2014).
Torbensen, K., Rossi, F., Ristori, S. & Abou-Hassan, A. Chemical communication and dynamics of droplet emulsions in networks of Belousov–Zhabotinsky micro-oscillators produced by microfluidics. Lab Chip 17, 1179–1189 (2017).
Ameta, S. et al. Darwinian properties and their trade-offs in autocatalytic RNA reaction networks. Nat. Commun. 12, 842 (2021).
Hu, J., Cochrane, W. G., Jones, A. X., Blackmond, D. G. & Paegel, B. M. Chiral lipid bilayers are enantioselectively permeable. Nat. Chem. 13, 786–791 (2021).
Wiedemeier, S. et al. Parametric studies on droplet generation reproducibility for applications with biological relevant fluids. Eng. Life Sci. 17, 1271–1280 (2017).
Kong, D. S. et al. Open-source, community-driven microfluidics with metafluidics. Nat. Biotechnol. 35, 523–529 (2017).
Becker, H. & Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 390, 89–111 (2008).
Guckenberger, D. J., Groot, T. E., de, Wan, A. M. D., Beebe, D. J. & Young, E. W. K. Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 15, 2364–2378 (2015).
Attia, U. M., Marson, S. & Alcock, J. R. Design and fabrication of a three-dimensional microfluidic device for blood separation using micro-injection moulding. Proc. Instn Mech. Engrs B J. Eng. Manuf. 228, 941–949 (2014).
Sugioka, K. et al. Femtosecond laser 3D micromachining: a powerful tool for the fabrication of microfluidic, optofluidic, and electrofluidic devices based on glass. Lab Chip 14, 3447–3458 (2014).
Waheed, S. et al. 3D printed microfluidic devices: enablers and barriers. Lab Chip 16, 1993–2013 (2016).
Lashkaripour, A., Silva, R. & Densmore, D. Desktop micromilled microfluidics. Microfluid. Nanofluidics 22, 31 (2018).
Plastic fantastic? Lab Chip 2, 31N–36N (2002).
Battat, S., Weitz, D. A. & Whitesides, G. M. An outlook on microfluidics: the promise and the challenge. Lab Chip 22, 530–536 (2022).
Xu, J. H., Li, S. W., Tan, J., Wang, Y. J. & Luo, G. S. Controllable preparation of monodisperse O/W and W/O emulsions in the same microfluidic device. Langmuir 22, 7943–7946 (2006).
Joensson, H. N., Uhlén, M. & Svahn, H. A. Droplet size based separation by deterministic lateral displacement — separating droplets by cell-induced shrinking. Lab Chip 11, 1305 (2011).
Volpatti, L. R. & Yetisen, A. K. Commercialization of microfluidic devices. Trends Biotechnol. 32, 347–350 (2014).
Reyes, D. R. et al. Accelerating innovation and commercialization through standardization of microfluidic-based medical devices. Lab Chip 21, 9–21 (2021).
Sanka, R., Lippai, J., Samarasekera, D., Nemsick, S. & Densmore, D. 3DμF-interactive design environment for continuous flow microfluidic devices. Sci. Rep. 9, 9166 (2019).
Elvira, K. S., Gielen, F., Tsai, S. S. H. & Nightingale, A. M. Materials and methods for droplet microfluidic device fabrication. Lab Chip 22, 859–875 (2022).
Tsur, E. E. Computer-aided design of microfluidic circuits. Annu. Rev. Biomed. Eng. 22, 285–307 (2020).
Walsh, D. I., Kong, D. S., Murthy, S. K. & Carr, P. A. Enabling microfluidics: from clean rooms to makerspaces. Trends Biotechnol. 35, 383–392 (2017).
Huebner, A. et al. Microdroplets: a sea of applications? Lab Chip 8, 1244–1254 (2008).
Amin, N., Thies, W. & Amarasinghe, S. Computer-aided design for microfluidic chips based on multilayer soft lithography. in Proc. 2009 IEEE International Conference on Computer Design 2–9 (IEEE, 2009).
Lashkaripour, A. et al. Machine learning enables design automation of microfluidic flow-focusing droplet generation. Nat. Commun. 12, 25 (2021).
Rosenfeld, L., Lin, T., Derda, R. & Tang, S. K. Y. Review and analysis of performance metrics of droplet microfluidics systems. Microfluid. Nanofluidics 16, 921–939 (2014).
Baret, J.-C., Kleinschmidt, F., El Harrak, A. & Griffiths, A. D. Kinetic aspects of emulsion stabilization by surfactants: a microfluidic analysis. Langmuir 25, 6088–6093 (2009).
Riechers, B. et al. Surfactant adsorption kinetics in microfluidics. Proc. Natl Acad. Sci. USA 113, 11465–11470 (2016).
Weiss, M. et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat. Mater. 17, 89–96 (2018).
Nassar, M. et al. Experimental models of the variation of HFE-7100 and HFE-7000 electric properties with temperature. IEEE Trans. Ind. Appl. 56, 4193–4199 (2020).
Ahn, K. et al. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 88, 024104 (2006).
Franke, T., Abate, A. R., Weitz, D. A. & Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 9, 2625–2627 (2009).
Brosseau, Q., Vrignon, J. & Baret, J.-C. Microfluidic dynamic interfacial tensiometry (μDIT). Soft Matter 10, 3066–3076 (2014).
Rosenfeld, L., Fan, L., Chen, Y., Swoboda, R. & Tang, S. K. Y. Break-up of droplets in a concentrated emulsion flowing through a narrow constriction. Soft Matter 10, 421–430 (2013).
Roach, L. S., Song, H. & Ismagilov, R. F. Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal. Chem. 77, 785–796 (2005).
Courtois, F. et al. Controlling the retention of small molecules in emulsion microdroplets for use in cell-based assays. Anal. Chem. 81, 3008–3016 (2009).
Skhiri, Y. et al. Dynamics of molecular transport by surfactants in emulsions. Soft Matter 8, 10618–10627 (2012).
Woronoff, G. et al. New generation of amino coumarin methyl sulfonate-based fluorogenic substrates for amidase assays in droplet-based microfluidic applications. Anal. Chem. 83, 2852–2857 (2011).
Janiesch, J.-W. et al. Key factors for stable retention of fluorophores and labeled biomolecules in droplet-based microfluidics. Anal. Chem. 87, 2063–2067 (2015).
Pan, M. et al. Fluorinated Pickering emulsions impede interfacial transport and form rigid interface for the growth of anchorage-dependent cells. ACS Appl. Mater. Interfaces 6, 21446–21453 (2014).
Chacon Orellana, L. A. & Baret, J.-C. Rapid stabilization of droplets by particles in microfluidics: role of droplet formation. ChemSystemsChem 1, 16–24 (2019).
Chacon, L. A. & Baret, J. C. Microfluidic angle of repose test for Pickering emulsions. J. Phys. Appl. Phys. 50, 39LT04 (2017).
Smith, C. A. et al. Sensitive, high throughput detection of proteins in individual, surfactant-stabilized picoliter droplets using nanoelectrospray ionization mass spectrometry. Anal. Chem. 85, 3812–3816 (2013).
Leduc, A., Huffman, R. G., Cantlon, J., Khan, S. & Slavov, N. Exploring functional protein covariation across single cells using nPOP. Genome Biol. 23, 261 (2022).
Alfaro, J. A. et al. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods 18, 604–617 (2021).
Brinkerhoff, H., Kang, A. S. W., Liu, J., Aksimentiev, A. & Dekker, C. Multiple rereads of single proteins at single–amino acid resolution using nanopores. Science 374, 1509–1513 (2021).
Chappell, L., Russell, A. J. C. & Voet, T. Single-cell (multi)omics technologies. Annu. Rev. Genomics Hum. Genet. 19, 15–41 (2018).
Lan, F., Haliburton, J. R., Yuan, A. & Abate, A. R. Droplet barcoding for massively parallel single-molecule deep sequencing. Nat. Commun. 7, 11784 (2016).
Chowdhury, M. S. et al. Dendronized fluorosurfactant for highly stable water-in-fluorinated oil emulsions with minimal inter-droplet transfer of small molecules. Nat. Commun. 10, 4546 (2019).
Pan, M., Lyu, F. & Tang, S. K. Y. Fluorinated Pickering emulsions with nonadsorbing interfaces for droplet-based enzymatic assays. Anal. Chem. 87, 7938–7943 (2015).
Shang, L. & Zhao, Y. Droplet-templated synthetic cells. Matter 4, 95–115 (2021).
Ugrinic, M., deMello, A. & Tang, T.-Y. D. Microfluidic tools for bottom-up synthetic cellularity. Chem 5, 1727–1742 (2019).
McIntyre, D., Lashkaripour, A. & Densmore, D. Rapid and inexpensive microfluidic electrode integration with conductive ink. Lab Chip 20, 3690–3695 (2020).
McIntyre, D., Lashkaripour, A., Fordyce, P. & Densmore, D. Machine learning for microfluidic design and control. Lab Chip 22, 2925–2937 (2022).
Utada, A. S., Fernandez-Nieves, A., Stone, H. A. & Weitz, D. A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 99, 094502 (2007).
Acknowledgements
The authors thank Y. Ding for assistance with the design and drawing of figures.
Author information
Authors and Affiliations
Contributions
Introduction (T.M. and A.J.d.); Experimentation (T.M., A.J.d. and J.-C.B.); Results (C.M. and A.R.A.); Applications (K.S., A.D.G., T.B., J.-C.B., T.M. and A.J.d.); Reproducibility and data deposition (D.A. and D.D.); Limitations and optimizations (T.B., J.-C.B., A.J.d., A.R.A., D.D. and A.D.G.); Outlook (A.J.d., A.R.A., J.-C.B., D.D. and A.D.G.); Overview of the Primer (T.M., A.J.d., A.R.A., J.-C.B., D.D. and A.D.G.).
Corresponding author
Ethics declarations
Competing interests
A.R.A. is a founder of Mission Bio and Fluent Bio. J.-C.B. is a founder and shareholder of Emulseo. D.D. is a founder and shareholder of Lattice Automation, Inc., Asimov Inc. and BioSens8 Inc. A.D.G. is a founder and shareholder of HiFiBiO Therapeutics, Biomillenia (now Design Pharmaceuticals), Cyprio and Minos Biosciences. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Heon-Ho Jeong, Melinda Simon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Affinity
-
The strength of the binding interaction between two molecules. Affinity can be described by the dissociation constant (KD) or by the standard free energy change (ΔG°): ΔG° = −RT ln KD where R is the gas constant and T is the absolute temperature.
- Bioprospection
-
A systematic and organized search for useful products derived from bioresources including plants, microorganisms and animals that can be developed further for commercialization or overall benefit to society.
- Capillary number
-
A dimensionless number used to quantify the ratio of viscous forces to capillary forces between two immiscible liquids.
- Colloidosomes
-
A solid microcapsule formed by the self-assembly of colloidal particles at the interface of emulsion droplets.
- Interfacial tension
-
The force of attraction between molecules at the interface of two fluids.
- In vitro transcription
-
Allows template-directed synthesis of bespoke RNA molecules in microgram to milligram quantities outside the cellular environment.
- In vitro transcription translation
-
Coupled in vitro transcription and in vitro translation allowing protein synthesis outside the cellular environment, thus enabling rapid expression of small amounts of functional proteins.
- Lentiviral libraries
-
Libraries of genes cloned into vectors derived from lentiviruses, which infect by inserting DNA into the host cell genome and which can infect non-dividing cells.
- Multi-omics
-
An analysis approach that combines data from multiple omic sources, such as genomics, proteomics, transcriptomics, epigenomics and metabolomics, to study living systems in a concerted manner.
- Opsonization
-
Opsonization is an immune process that uses opsonins (extracellular proteins) to mark foreign pathogens for elimination by phagocytes.
- Phage display
-
A method to select large libraries of genes encoding proteins, in which genes are inserted into a phage coat protein gene, resulting in phage particles with the protein displayed on the surface and the gene that encodes it inside the phage particle, generating a connection between genotype and phenotype.
- Poisson loading
-
An encapsulation strategy in which droplet occupancy follows a Poisson distribution.
- Polymersomes
-
An artificial vesicle in which the vesicle membrane is composed of amphiphilic block or triblock copolymers, with high stability and tunable size.
- Reynolds number
-
A dimensionless parameter quantifying the ratio of inertial forces to viscous forces in a system, useful in predicting whether a flow will be laminar or turbulent.
- Taylor cone
-
The shape of a fluid jet generated during electrospraying (such as during the sample ionization for mass spectrometry).
- Taylor dispersion
-
An effect in which shear acts to smear out the concentration distribution in the direction of the flow, enhancing the rate at which it spreads in that direction.
- Wettability
-
Describes the ability of a liquid to spread over a surface. It is normally quantified through measurement of the contact angle between the liquid and the surface.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moragues, T., Arguijo, D., Beneyton, T. et al. Droplet-based microfluidics. Nat Rev Methods Primers 3, 32 (2023). https://doi.org/10.1038/s43586-023-00212-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00212-3
This article is cited by
-
Convergence of machine learning with microfluidics and metamaterials to build smart materials
International Journal on Interactive Design and Manufacturing (IJIDeM) (2024)
-
Tröpfchenmikrofluidik für das Enzymscreening
BIOspektrum (2024)