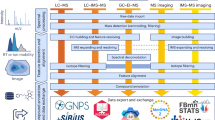
Abstract
Peptides are biopolymers, typically consisting of 2–50 amino acids. They are biologically produced by the cellular ribosomal machinery or by non-ribosomal enzymes and, sometimes, other dedicated ligases. Peptides are arranged as linear chains or cycles, and include post-translational modifications, unusual amino acids and stabilizing motifs. Their structure and molecular size render them a unique chemical space, between small molecules and larger proteins. Peptides have important physiological functions as intrinsic signalling molecules, such as neuropeptides and peptide hormones, for cellular or interspecies communication, as toxins to catch prey or as defence molecules to fend off enemies and microorganisms. Clinically, they are gaining popularity as biomarkers or innovative therapeutics; to date there are more than 60 peptide drugs approved and more than 150 in clinical development. The emerging field of peptidomics comprises the comprehensive qualitative and quantitative analysis of the suite of peptides in a biological sample (endogenously produced, or exogenously administered as drugs). Peptidomics employs techniques of genomics, modern proteomics, state-of-the-art analytical chemistry and innovative computational biology, with a specialized set of tools. The complex biological matrices and often low abundance of analytes typically examined in peptidomics experiments require optimized sample preparation and isolation, including in silico analysis. This Primer covers the combination of techniques and workflows needed for peptide discovery and characterization and provides an overview of various biological and clinical applications of peptidomics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gruber, C. W., Muttenthaler, M. & Freissmuth, M. Ligand-based peptide design and combinatorial peptide libraries to target G protein-coupled receptors. Curr. Pharm. Des. 16, 3071–3088 (2010).
Dang, T. & Süssmuth, R. D. Bioactive peptide natural products as lead structures for medicinal use. Acc. Chem. Res. 50, 1566–1576 (2017).
Fosgerau, K. & Hoffmann, T. Peptide therapeutics: current status and future directions. Drug Discov. Today 20, 122–128 (2015).
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021). This comprehensive review discusses the importance of peptides as drug leads and innovative therapeutics.
Craik, D. J., Fairlie, D. P., Liras, S. & Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 81, 136–147 (2013).
Munch, J., Standker, L., Forssmann, W. G. & Kirchhoff, F. Discovery of modulators of HIV-1 infection from the human peptidome. Nat. Rev. Microbiol. 12, 715–722 (2014).
Baggerman, G. et al. Peptidomics. J. Chromatogr. B 803, 3–16 (2004).
Schrader, M., Schulz-Knappe, P. & Fricker, L. D. Historical perspective of peptidomics. EuPA Open. Proteom. 3, 171–182 (2014).
Schulz-Knappe, P. et al. Peptidomics: the comprehensive analysis of peptides in complex biological mixtures. Comb. Chem. High. T Scr. 4, 207–217 (2001).
Metrano, A. J. et al. Asymmetric catalysis mediated by synthetic peptides, version 2.0: expansion of scope and mechanisms. Chem. Rev. 120, 11479–11615 (2020). This review article discusses peptide-assisted asymmetric synthesis reactions and recent advances in the field.
Collier, J. H. & Segura, T. Evolving the use of peptides as components of biomaterials. Biomaterials 32, 4198–4204 (2011).
Agnieray, H., Glasson, J. L., Chen, Q., Kaur, M. & Domigan, L. J. Recent developments in sustainably sourced protein-based biomaterials. Biochem. Soc. Trans. 49, 953–964 (2021).
Malandrino, N. & Smith, R. J. in Principles of Endocrinology and Hormone Action (eds Belfiore, A. & LeRoith, D.) 29–42 (Springer International, 2018).
Yi, J., Warunek, D. & Craft, D. Degradation and stabilization of peptide hormones in human blood specimens. PLoS ONE 10, e0134427 (2015).
Svensson, M. et al. Heat stabilization of the tissue proteome: a new technology for improved proteomics. J. Proteome Res. 8, 974–981 (2009).
Yang, N., Anapindi, K. D. B., Romanova, E. V., Rubakhin, S. S. & Sweedler, J. V. Improved identification and quantitation of mature endogenous peptides in the rodent hypothalamus using a rapid conductive sample heating system. Analyst 142, 4476–4485 (2017).
Feist, P. & Hummon, A. B. Proteomic challenges: sample preparation techniques for microgram-quantity protein analysis from biological samples. Int. J. Mol. Sci. 16, 3537–3563 (2015).
Harrison, S. T. Bacterial cell disruption: a key unit operation in the recovery of intracellular products. Biotechnol. Adv. 9, 217–240 (1991).
Koehbach, J. et al. Cyclotide discovery in Gentianales revisited — identification and characterization of cyclic cystine-knot peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers 100, 438–452 (2013).
Chen, E. I., Cociorva, D., Norris, J. L. & Yates, J. R. 3rd Optimization of mass spectrometry-compatible surfactants for shotgun proteomics. J. Proteome Res. 6, 2529–2538 (2007).
Panuwet, P. et al. Biological matrix effects in quantitative tandem mass spectrometry-based analytical methods: advancing biomonitoring. Crit. Rev. Anal. Chem. 46, 93–105 (2016).
Finoulst, I., Pinkse, M., Van Dongen, W. & Verhaert, P. Sample preparation techniques for the untargeted LC-MS-based discovery of peptides in complex biological matrices. J. Biomed. Biotechnol. 2011, 245291 (2011).
Tubaon, R. M., Haddad, P. R. & Quirino, J. P. Sample clean-up strategies for ESI mass spectrometry applications in bottom-up proteomics: trends from 2012 to 2016. Proteomics 17, 1700011 (2017).
Sosalagere, C., Adesegun Kehinde, B. & Sharma, P. Isolation and functionalities of bioactive peptides from fruits and vegetables: a reviews. Food Chem. 366, 130494 (2022).
Mthembu, S. N., Sharma, A., Albericio, F. & de la Torre, B. G. Breaking a couple: disulfide reducing agents. Chembiochem 21, 1947–1954 (2020).
Hellinger, R. et al. Importance of the cyclic cystine knot structural motif for immunosuppressive effects of cyclotides. ACS Chem. Biol. 16, 2373–2386 (2021).
Tsai, P. L., Chen, S. F. & Huang, S. Y. Mass spectrometry-based strategies for protein disulfide bond identification. Rev. Anal. Chem. 32, 257–268 (2013).
Han, D. K., Eng, J., Zhou, H. & Aebersold, R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat. Biotechnol. 19, 946–951 (2001).
Yao, X., Freas, A., Ramirez, J., Demirev, P. A. & Fenselau, C. Proteolytic 18O labeling for comparative proteomics: model studies with two serotypes of adenovirus. Anal. Chem. 73, 2836–2842 (2001).
Hsu, J.-L., Huang, S.-Y., Chow, N.-H. & Chen, S.-H. Stable-isotope dimethyl labeling for quantitative proteomics. Anal. Chem. 75, 6843–6852 (2003).
Greer, T., Lietz, C. B., Xiang, F. & Li, L. Novel isotopic N,N-dimethyl leucine (iDiLeu) reagents enable absolute quantification of peptides and proteins using a standard curve approach. J. Am. Soc. Mass Spectrom. 26, 107–119 (2014).
DeSouza, L. V. et al. Multiple reaction monitoring of mTRAQ-labeled peptides enables absolute quantification of endogenous levels of a potential cancer marker in cancerous and normal endometrial tissues. J. Proteome Res. 7, 3525–3534 (2008).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Wiese, S., Reidegeld, K. A., Meyer, H. E. & Warscheid, B. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics 7, 340–350 (2007).
Zhang, J., Wang, Y. & Li, S. Deuterium isobaric amine-reactive tags for quantitative proteomics. Anal. Chem. 82, 7588–7595 (2010).
Atkins, N. Jr. et al. Functional peptidomics: stimulus- and time-of-day-specific peptide release in the mammalian circadian clock. ACS Chem. Neurosci. 9, 2001–2008 (2018).
Gedela, S. & Medicherla, N. R. Chromatographic techniques for the separation of peptides: application to proteomics. Chromatographia 65, 511–518 (2007).
Udeshi, N. D., Compton, P. D., Shabanowitz, J., Hunt, D. F. & Rose, K. L. Methods for analyzing peptides and proteins on a chromatographic timescale by electron-transfer dissociation mass spectrometry. Nat. Protoc. 3, 1709–1717 (2008).
Mahoney, W. C. & Hermodson, M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J. Biol. Chem. 255, 11199–11203 (1980).
Yoshida, T. Peptide separation by hydrophilic-interaction chromatography: a review. J. Biochem. Biophys. Meth. 60, 265–280 (2004).
Hillenkamp, F. & Karas, M. Mass spectrometry of peptides and proteins by matrix-assisted ultraviolet laser desorption/ionization. Meth. Enzymol. 193, 280–295 (1990).
Dreisewerd, K. The desorption process in MALDI. Chem. Rev. 103, 395–426 (2003).
Dong, X. et al. A LC-MS/MS method to monitor the concentration of HYD-PEP06, a RGD-modified Endostar mimetic peptide in rat blood. J. Chromatogr. B 1092, 296–305 (2018).
Lange, V., Picotti, P., Domon, B. & Aebersold, R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 4, 222 (2008).
Ludwig, C. et al. Data-independent acquisition-based SWATH-MS for quantitative proteomics: a tutorial. Mol. Syst. Biol. 14, e8126 (2018).
Gerber, S. A., Rush, J., Stemman, O., Kirschner, M. W. & Gygi, S. P. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc. Natl Acad. Sci. USA 100, 6940–6945 (2003).
Follmann, R., Goldsmith, C. J. & Stein, W. Spatial distribution of intermingling pools of projection neurons with distinct targets: a 3D analysis of the commissural ganglia in Cancer borealis. J. Comp. Neurol. 525, 1827–1843 (2017).
Mechref, Y. Use of CID/ETD mass spectrometry to analyze glycopeptides. Curr. Protoc. Protein Sci. 68, 12.11.1–12.11.11 (2012).
Riley, N. M., Malaker, S. A., Driessen, M. D. & Bertozzi, C. R. Optimal dissociation methods differ for N-and O-glycopeptides. J. Proteome Res. 19, 3286–3301 (2020).
Demichev, V., Messner, C. B., Vernardis, S. I., Lilley, K. S. & Ralser, M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 17, 41–44 (2020).
Tsou, C.-C. et al. DIA-Umpire: comprehensive computational framework for data-independent acquisition proteomics. Nat. Methods 12, 258–264 (2015).
Sinitcyn, P. et al. MaxDIA enables library-based and library-free data-independent acquisition proteomics. Nat. Biotechnol. https://doi.org/10.1038/s41587-021-00968-7 (2021).
Caprioli, R. M., Farmer, T. B. & Gile, J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751–4760 (1997).
Tyler, B. J., Rayal, G. & Castner, D. G. Multivariate analysis strategies for processing ToF-SIMS images of biomaterials. Biomaterials 28, 2412–2423 (2007).
Eberlin, L. S. et al. Desorption electrospray ionization then MALDI mass spectrometry imaging of lipid and protein distributions in single tissue sections. Anal. Chem. 83, 8366–8371 (2011).
Bouschen, W. & Spengler, B. Artifacts of MALDI sample preparation investigated by high-resolution scanning microprobe matrix-assisted laser desorption/ionization (SMALDI) imaging mass spectrometry. Int. J. Mass. Spectrom. 266, 129–137 (2007).
Iakab, S.-A. et al. SALDI-MS and SERS multimodal imaging: one nanostructured substrate to rule them both. Anal. Chem. 94, 2785–2793 (2022).
Ali, A., Baby, B., Soman, S. S. & Vijayan, R. Molecular insights into the interaction of hemorphin and its targets. Sci. Rep. 9, 14747 (2019).
Rocha, B., Ruiz-Romero, C. & Blanco, F. J. Mass spectrometry imaging: a novel technology in rheumatology. Nat. Rev. Rheumatol. 13, 52–63 (2017).
Ramos-Vara, J. Technical aspects of immunohistochemistry. Vet. Pathol. 42, 405–426 (2005).
Skelley, D., Brown, L. & Besch, P. Radioimmunoassay. Clin. Chem. 19, 146–186 (1973).
Lichtman, J. W. & Conchello, J.-A. Fluorescence microscopy. Nat. Methods 2, 910–919 (2005).
Buchberger, A. R., DeLaney, K., Johnson, J. & Li, L. Mass spectrometry imaging: a review of emerging advancements and future insights. Anal. Chem. 90, 240 (2018). This comprehensive review discusses various aspects of MSI, spanning from sample preparation and mass spectrometry instrumentation to data analysis and diverse applications.
Lemaire, R. et al. Direct analysis and MALDI imaging of formalin-fixed, paraffin-embedded tissue sections. J. Proteome Res. 6, 1295–1305 (2007).
Kokkat, T. J. et al. Archived formalin-fixed paraffin-embedded (FFPE) blocks: a valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv. Biobank 11, 101–106 (2013).
Ren, Y. et al. Reagents for isobaric labeling peptides in quantitative proteomics. Anal. Chem. 90, 12366–12371 (2018).
Truong, J. X. et al. Removal of optimal cutting temperature (OCT) compound from embedded tissue for MALDI imaging of lipids. Anal. Bioanal. Chem. 413, 2695–2708 (2021).
Tian, Y., Bova, G. S. & Zhang, H. Quantitative glycoproteomic analysis of optimal cutting temperature-embedded frozen tissues identifying glycoproteins associated with aggressive prostate cancer. Anal. Chem. 83, 7013–7019 (2011).
Bogdanow, B., Zauber, H. & Selbach, M. Systematic errors in peptide and protein identification and quantification by modified peptides. Mol. Cell Proteom. 15, 2791–2801 (2016).
Schwartz, S. A., Reyzer, M. L. & Caprioli, R. M. Direct tissue analysis using matrix‐assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J. Mass. Spectrom. 38, 699–708 (2003).
Lemaire, R. et al. MALDI-MS direct tissue analysis of proteins: improving signal sensitivity using organic treatments. Anal. Chem. 78, 7145–7153 (2006).
Buchberger, A. R., Sauer, C. S., Vu, N. Q., DeLaney, K. & Li, L. Temporal study of the perturbation of crustacean neuropeptides due to severe hypoxia using 4-plex reductive dimethylation. J. Proteome Res. 19, 1548–1555 (2020).
Kaletaş, B. K. et al. Sample preparation issues for tissue imaging by imaging MS. Proteomics 9, 2622–2633 (2009).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Leopold, J., Popkova, Y., Engel, K. M. & Schiller, J. Recent developments of useful MALDI matrices for the mass spectrometric characterization of lipids. Biomolecules 8, 173 (2018).
DeLaney, K. et al. Mass spectrometry quantification, localization, and discovery of feeding-related neuropeptides in cancer borealis. ACS Chem. Neurosci. 12, 782–798 (2021).
Amos, B. et al. VEuPathDB: the eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 50, D898–D911 (2022).
Sayers, E. W. et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50, D20–D26 (2022).
The UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Kaas, Q., Yu, R., Jin, A. H., Dutertre, S. & Craik, D. J. ConoServer: updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 40, D325–D330 (2012).
Kautsar, S. A. et al. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 48, D454–D458 (2020).
Leinonen, R. et al. The European Nucleotide Archive. Nucleic Acids Res. 39, D28–D31 (2011).
Besemer, J. & Borodovsky, M. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res. 33, W451–W454 (2005).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Hazarika, R. R. et al. ARA-PEPs: a repository of putative sORF-encoded peptides in Arabidopsis thaliana. BMC Bioinformatics 18, 37 (2017).
Mooney, C., Haslam, N. J., Holton, T. A., Pollastri, G. & Shields, D. C. PeptideLocator: prediction of bioactive peptides in protein sequences. Bioinformatics 29, 1120–1126 (2013).
Zhou, P. et al. Detecting small plant peptides using SPADA (Small Peptide Alignment Discovery Application). BMC Bioinformatics 14, 335 (2013).
Zhu, M. & Gribskov, M. MiPepid: microPeptide identification tool using machine learning. BMC Bioinformatics 20, 559 (2019).
Zhang, Y., Jia, C., Fullwood, M. J. & Kwoh, C. K. DeepCPP: a deep neural network based on nucleotide bias information and minimum distribution similarity feature selection for RNA coding potential prediction. Brief. Bioinform. 22, 2073–2084 (2021).
Lin, D. et al. Mining amphibian and insect transcriptomes for antimicrobial peptide sequences with rAMPage. Antibiotics 11, 952 (2022).
Lyons, E. & Freeling, M. How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53, 661–673 (2008).
Dieckmann, M. A. et al. EDGAR3.0: comparative genomics and phylogenomics on a scalable infrastructure. Nucleic Acids Res. 49, W185–W192 (2021).
Medema, M. H. & Fischbach, M. A. Computational approaches to natural product discovery. Nat. Chem. Biol. 11, 639–648 (2015).
Weber, T. & Kim, H. U. The secondary metabolite bioinformatics portal: computational tools to facilitate synthetic biology of secondary metabolite production. Synth. Syst. Biotechnol. 1, 69–79 (2016).
Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87 (2019). This work presents the most significant genome mining platform for natural products, covering a wide range of compounds, and is a recommended read for anyone interested in natural product research.
Hannigan, G. D. et al. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 47, e110 (2019).
Sélem-Mojica, N., Aguilar, C., Gutiérrez-García, K., Martínez-Guerrero, C. E. & Barona-Gómez, F. EvoMining reveals the origin and fate of natural product biosynthetic enzymes. Microb. Genom. https://doi.org/10.1099/mgen.0.000260 (2019).
Chevrette, M. G., Aicheler, F., Kohlbacher, O., Currie, C. R. & Medema, M. H. SANDPUMA: ensemble predictions of nonribosomal peptide chemistry reveal biosynthetic diversity across Actinobacteria. Bioinformatics 33, 3202–3210 (2017). This work discusses how SANDPUMA has aided NRP discovery and continues to provide valuable predictions for researchers involved in NRP research.
van Heel, A. J. et al. BAGEL4: a user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 46, W278–W281 (2018).
Ramesh, S. et al. Bioinformatics-guided expansion and discovery of graspetides. ACS Chem. Biol. 16, 2787–2797 (2021).
Merwin, N. J. et al. DeepRiPP integrates multiomics data to automate discovery of novel ribosomally synthesized natural products. Proc. Natl Acad. Sci. USA 117, 371–380 (2020).
Schlaffner, C. N., Pirklbauer, G. J., Bender, A. & Choudhary, J. S. Fast, quantitative and variant enabled mapping of peptides to genomes. Cell Syst. 5, 152–156.e4 (2017).
Ricart, E. et al. rBAN: retro-biosynthetic analysis of nonribosomal peptides. J. Cheminform. 11, 13 (2019).
Kunyavskaya, O. et al. Nerpa: a tool for discovering biosynthetic gene clusters of bacterial nonribosomal peptides. Metabolites https://doi.org/10.3390/metabo11100693 (2021).
Konanov, D. N., Krivonos, D. V., Ilina, E. N. & Babenko, V. V. BioCAT: search for biosynthetic gene clusters producing nonribosomal peptides with known structure. Comput. Struct. Biotechnol. J. 20, 1218–1226 (2022).
Grundemann, C., Koehbach, J., Huber, R. & Gruber, C. W. Do plant cyclotides have potential as immunosuppressant peptides? J. Nat. Prod. 75, 167–174 (2012).
van Santen, J. A. et al. The Natural Products Atlas: an open access knowledge base for microbial natural products discovery. ACS Cent. Sci. 5, 1824–1833 (2019). This work discusses how the Natural Products Atlas provides valuable information, visualization and validation of discovered compounds.
Mohimani, H. et al. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 9, 4035 (2018).
Diament, B. J. & Noble, W. S. Faster SEQUEST searching for peptide identification from tandem mass spectra. J. Proteome Res. 10, 3871–3879 (2011).
Pluskal, T., Castillo, S., Villar-Briones, A. & Oresic, M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11, 395 (2010).
Claesen, J., Valkenborg, D. & Burzykowski, T. De novo prediction of the elemental composition of peptides and proteins based on a single mass. J. Mass. Spectrom. 55, e4367 (2020).
Lai, Z. et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 15, 53–56 (2018).
Palmer, A. et al. FDR-controlled metabolite annotation for high-resolution imaging mass spectrometry. Nat. Methods 14, 57–60 (2017).
Novak, J., Skriba, A. & Havlicek, V. CycloBranch 2: molecular formula annotations applied to imzML data sets in bimodal fusion and LC-MS data files. Anal. Chem. 92, 6844–6849 (2020).
Ricart, E., Pupin, M., Muller, M. & Lisacek, F. Automatic annotation and dereplication of tandem mass spectra of peptidic natural products. Anal. Chem. 92, 15862–15871 (2020).
Gurevich, A. et al. Increased diversity of peptidic natural products revealed by modification-tolerant database search of mass spectra. Nat. Microbiol. 3, 319–327 (2018).
Seidler, J., Zinn, N., Boehm, M. E. & Lehmann, W. D. De novo sequencing of peptides by MS/MS. Proteomics 10, 634–649 (2010).
Yang, H., Chi, H., Zeng, W.-F., Zhou, W.-J. & He, S.-M. pNovo 3: precise de novo peptide sequencing using a learning-to-rank framework. Bioinformatics 35, i183–i190 (2019).
Tran, N. H. et al. Deep learning enables de novo peptide sequencing from data-independent-acquisition mass spectrometry. Nat. Methods 16, 63–66 (2019).
Tran, N. H., Zhang, X., Xin, L., Shan, B. & Li, M. De novo peptide sequencing by deep learning. Proc. Natl Acad. Sci. USA 114, 8247–8252 (2017).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for mass spectrometry-based proteomics. Meth. Mol. Biol. 604, 55–71 (2010).
Reiter, L. et al. Protein identification false discovery rates for very large proteomics data sets generated by tandem mass spectrometry. Mol. Cell Proteom. 8, 2405–2417 (2009).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Perkins, D. N., Pappin, D. J. C., Creasy, D. M. & Cottrell, J. S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20, 3551–3567 (1999).
MacLean, B. et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 (2010).
Röst, H. L. et al. OpenSWATH enables automated, targeted analysis of data-independent acquisition MS data. Nat. Biotechnol. 32, 219–223 (2014).
Bruderer, R. et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteom. 14, 1400–1410 (2015).
Neilson, K. A. et al. Less label, more free: approaches in label‐free quantitative mass spectrometry. Proteomics 11, 535–553 (2011).
Chang, C. et al. LFAQ: toward unbiased label-free absolute protein quantification by predicting peptide quantitative factors. Anal. Chem. 91, 1335–1343 (2018).
Ishihama, Y. et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteom. 4, 1265–1272 (2005).
Sivanich, M. K., Gu, T. J., Tabang, D. N. & Li, L. Recent advances in isobaric labeling and applications in quantitative proteomics. Proteomics 22, e2100256 (2022). This critical review article discusses isobaric labelling strategies for quantitative proteomics and peptidomics applications as well as current limitations and future outlooks.
Fonville, J. M. et al. Robust data processing and normalization strategy for MALDI mass spectrometric imaging. Anal. Chem. 84, 1310–1319 (2012).
Deininger, S.-O. et al. Normalization in MALDI-TOF imaging datasets of proteins: practical considerations. Anal. Bioanal. Chem. 401, 167–181 (2011).
Källback, P., Shariatgorji, M., Nilsson, A. & Andrén, P. E. Novel mass spectrometry imaging software assisting labeled normalization and quantitation of drugs and neuropeptides directly in tissue sections. J. Proteom. 75, 4941–4951 (2012).
Shariatgorji, M. et al. Direct targeted quantitative molecular imaging of neurotransmitters in brain tissue sections. Neuron 84, 697–707 (2014).
Lanekoff, I., Thomas, M. & Laskin, J. Shotgun approach for quantitative imaging of phospholipids using nanospray desorption electrospray ionization mass spectrometry. Anal. Chem. 86, 1872–1880 (2014).
Hansen, H. T. & Janfelt, C. Aspects of quantitation in mass spectrometry imaging investigated on cryo-sections of spiked tissue homogenates. Anal. Chem. 88, 11513–11520 (2016).
Robichaud, G., Garrard, K. P., Barry, J. A. & Muddiman, D. C. MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J. Am. Soc. Mass. Spectrom. 24, 718–721 (2013).
Alexander, J., Oliphant, A., Wilcockson, D. C. & Webster, S. G. Functional identification and characterization of the diuretic hormone 31 (DH31) signaling system in the green shore crab, Carcinus maenas. Front. Neurosci. 12, 454 (2018).
Källback, P., Nilsson, A., Shariatgorji, M. & Andrén, P. E. msIQuant — quantitation software for mass spectrometry imaging enabling fast access, visualization, and analysis of large data sets. Anal. Chem. 88, 4346–4353 (2016).
Arnison, P. G. et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Product. Rep. 30, 108–160 (2013). This comprehensive review introduces the reader to RiPPs, from classification to biosynthesis and bioactivity.
Wiebach, V. et al. The anti-staphylococcal lipolanthines are ribosomally synthesized lipopeptides. Nat. Chem. Biol. 14, 652–654 (2018). This research article discusses a novel type of anti-staphylococcal RiPP, utilizing a short peptide conjugated with a lipid moiety.
Sussmuth, R. D. & Mainz, A. Nonribosomal peptide synthesis-principles and prospects. Angew. Chem. Int. Ed. 56, 3770–3821 (2017). This comprehensive review about NRPs explains biosynthesis, structures and bioactivity or NRPs.
Tang, S. et al. Discovery and characterization of a PKS-NRPS hybrid in Aspergillus terreus by genome mining. J. Nat. Prod. 83, 473–480 (2020).
Zhang, Z., Wang, J., Wang, J., Wang, J. & Li, Y. Estimate of the sequenced proportion of the global prokaryotic genome. Microbiome https://doi.org/10.1186/s40168-020-00903-z (2020).
V, V. et al. Venom peptides — a comprehensive translational perspective in pain management. Curr. Res. Toxicol. 2, 329–340 (2021).
King, G. F. & Hardy, M. C. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 58, 475–496 (2013).
Munawar, A., Ali, S. A., Akrem, A. & Betzel, C. Snake venom peptides: tools of biodiscovery. Toxins https://doi.org/10.3390/toxins10110474 (2018).
King, G. F. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert. Opin. Biol. Ther. 11, 1469–1484 (2011).
Dutertre, S. et al. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 5, 3521 (2014). This research article investigates the differences between the defensive and predatory venoms of cone snails.
Prashanth, J. R., Dutertre, S. & Lewis, R. J. in Evolution of Venomous Animals and Their Toxins Ch. 18 (ed. Malhotra, A.) 105–123 (Springer, 2017).
Coelho, P., Kaliontzopoulou, A., Rasko, M., Meijden, A. & Portugal, S. A ‘striking’ relationship: scorpion defensive behaviour and its relation to morphology and performance. Funct. Ecol. 31, 1390–1404 (2017). This work presents a fascinating investigation into the different methods of the defensive behaviours of scorpions, measuring both the speed and frequency of stings in response to stimuli.
Nisani, Z. & Hayes, W. K. Defensive stinging by Parabuthus transvaalicus scorpions: risk assessment and venom metering. Anim. Behav. 81, 627–633 (2011).
Diesner, M., Predel, R. & Neupert, S. Neuropeptide mapping of dimmed cells of adult Drosophila brain. J. Am. Soc. Mass. Spectrom. 29, 890–902 (2018).
Habenstein, J. et al. Transcriptomic, peptidomic, and mass spectrometry imaging analysis of the brain in the ant Cataglyphis nodus. J. Neurochem. 158, 391–412 (2021).
Zeng, H. et al. Genomics- and peptidomics-based discovery of conserved and novel neuropeptides in the American cockroach. J. Proteome Res. 20, 1217–1228 (2021).
El Filali, Z., Van Minnen, J., Liu, W. K., Smit, A. B. & Li, K. W. Peptidomics analysis of neuropeptides involved in copulatory behavior of the mollusk Lymnaea stagnalis. J. Proteome Res. 5, 1611–1617 (2006).
Parmar, B. S. et al. Identification of non-canonical translation products in C. elegans using tandem mass spectrometry. Front. Genet. 12, 728900 (2021).
Van Bael, S. et al. A Caenorhabditis elegans mass spectrometric resource for neuropeptidomics. J. Am. Soc. Mass. Spectrom. 29, 879–889 (2018).
Wood, E. A. et al. Neuropeptide localization in Lymnaea stagnalis: from the central nervous system to subcellular compartments. Front. Mol. Neurosci. 14, 670303 (2021).
DeLaney, K., Buchberger, A. & Li, L. Identification, quantitation, and imaging of the crustacean peptidome. Methods Mol. Biol. 1719, 247–269 (2018).
DeLaney, K. & Li, L. Capillary electrophoresis coupled to MALDI mass spectrometry imaging with large volume sample stacking injection for improved coverage of C. borealis neuropeptidome. Analyst 145, 61–69 (2019).
Liu, Y., Li, G. & Li, L. Targeted top-down mass spectrometry for the characterization and tissue-specific functional discovery of crustacean hyperglycemic hormones (CHH) and CHH precursor-related peptides in response to low pH stress. J. Am. Soc. Mass. Spectrom. 32, 1352–1360 (2021).
Xu, L. L. et al. Major shrimp allergen peptidomics signatures and potential biomarkers of heat processing. Food Chem. 382, 132567 (2022).
Phetsanthad, A. et al. Recent advances in mass spectrometry analysis of neuropeptides. Mass. Spectrom. Rev. 42, 706–750 (2021).
Fujisawa, T. & Hayakawa, E. Peptide signaling in Hydra. Int. J. Dev. Biol. 56, 543–550 (2012).
Monroe, E. B. et al. Exploring the sea urchin neuropeptide landscape by mass spectrometry. J. Am. Soc. Mass. Spectrom. 29, 923–934 (2018).
Takahashi, T. Neuropeptides and epitheliopeptides: structural and functional diversity in an ancestral metazoan Hydra. Protein Pept. Lett. 20, 671–680 (2013).
Southey, B. R., Romanova, E. V., Rodriguez-Zas, S. L. & Sweedler, J. V. Bioinformatics for prohormone and neuropeptide discovery. Methods Mol. Biol. 1719, 71–96 (2018). This methodological article describes a pipeline for annotation of neuropeptide prohormones from genomic assemblies using freely available public toolsets and databases.
Hu, C. K. et al. Identification of prohormones and pituitary neuropeptides in the African cichlid, Astatotilapia burtoni. BMC Genomics 17, 660 (2016).
Chan-Andersen, P. C., Romanova, E. V., Rubakhin, S. S. & Sweedler, J. V. Profiling 26,000 Aplysia californica neurons by single cell mass spectrometry reveals neuronal populations with distinct neuropeptide profiles. J. Biol. Chem. 298, 102254 (2022). This work presents an elegant mass spectrometry-based approach for robust categorization of large cell populations based on a single-cell neuropeptide profile.
Jiménez, C. R. et al. Peptidomics of a single identified neuron reveals diversity of multiple neuropeptides with convergent actions on cellular excitability. J. Neurosci. 26, 518–529 (2006).
Green, D. J. et al. cAMP, Ca2+, pHi, and NO regulate H-like cation channels that underlie feeding and locomotion in the predatory sea slug Pleurobranchaea californica. ACS Chem. Neurosci. 9, 1986–1993 (2018).
Han, Y., Ma, B. & Zhang, K. SPIDER: software for protein identification from sequence tags with de novo sequencing error. J. Bioinform. Comput. Biol. 3, 697–716 (2005).
Romanova, E. V., Aerts, J. T., Croushore, C. A. & Sweedler, J. V. Small-volume analysis of cell-cell signaling molecules in the brain. Neuropsychopharmacology 39, 50–64 (2014).
Bai, L. et al. Characterization of GdFFD, a d-amino acid-containing neuropeptide that functions as an extrinsic modulator of the Aplysia feeding circuit. J. Biol. Chem. 288, 32837–32851 (2013).
Checco, J. W. et al. Aplysia allatotropin-related peptide and its newly identified d-amino acid-containing epimer both activate a receptor and a neuronal target. J. Biol. Chem. 293, 16862–16873 (2018).
Romanova, E. V. et al. Urotensin II in invertebrates: from structure to function in Aplysia californica. PLoS ONE 7, e48764 (2012).
Zhang, G. et al. Newly identified Aplysia SPTR-gene family-derived peptides: localization and function. ACS Chem. Neurosci. 9, 2041–2053 (2018).
Mast, D. H., Checco, J. W. & Sweedler, J. V. Differential post-translational amino acid isomerization found among neuropeptides in Aplysia californica. ACS Chem. Biol. 15, 272–281 (2020).
Mast, D. H., Checco, J. W. & Sweedler, J. V. Advancing d-amino acid-containing peptide discovery in the metazoan. Biochim. Biophys. Acta Proteins Proteom. 1869, 140553 (2021). This review discusses the prevalence of enzyme-derived DAACPs among animals, physiological consequences of peptide isomerization and analytical methods for structural characterization/discovery of DAACPs.
Lambeth, T. R. & Julian, R. R. Differentiation of peptide isomers and epimers by radical-directed dissociation. Methods Enzymol. 626, 67–87 (2019).
Mast, D. H., Liao, H. W., Romanova, E. V. & Sweedler, J. V. Analysis of peptide stereochemistry in single cells by capillary electrophoresis-trapped ion mobility spectrometry mass spectrometry. Anal. Chem. 93, 6205–6213 (2021).
Checco, J. W. et al. Molecular and physiological characterization of a receptor for d-amino acid-containing neuropeptides. ACS Chem. Biol. 13, 1343–1352 (2018).
Livnat, I. et al. A d-amino acid-containing neuropeptide discovery funnel. Anal. Chem. 88, 11868–11876 (2016).
Yussif, B. M. & Checco, J. W. Evaluation of endogenous peptide stereochemistry using liquid chromatography-mass spectrometry-based spiking experiments. Methods Enzymol. 663, 205–234 (2022).
Jiang, L. et al. A quantitative proteome map of the human body. Cell 183, 269–283.e19 (2020).
Secher, A. et al. Analytic framework for peptidomics applied to large-scale neuropeptide identification. Nat. Commun. 7, 11436 (2016). This article introduces a comprehensive analytical workflow for large-scale mammalian peptidomics studies, detailing procedures ranging from sample preparation to data analysis.
Foster, S. R. et al. Discovery of human signaling systems: pairing peptides to G protein-coupled receptors. Cell 179, 895–908.e21 (2019).
Hauser, A. S., Gloriam, D. E., Brauner-Osborne, H. & Foster, S. R. Novel approaches leading towards peptide GPCR de-orphanisation. Br. J. Pharmacol. 177, 961–968 (2020).
Scarpa, A. Pre-scientific medicines: their extent and value. Soc. Sci. Med. A Med. Psychol. Med. Sociol. 15, 317–326 (1981).
Pina, A. S., Hussain, A. & Roque, A. C. A. in Ligand–Macromolecular Interactions in Drug Discovery: Methods and Protocols (ed. Roque, A. C. A.) 3–12 (Humana, 2010).
Heinrich, M. Ethnobotany and its role in drug development. Phytother. Res. 14, 479–488 (2000).
Campbell, I. B., Macdonald, S. J. F. & Procopiou, P. A. Medicinal chemistry in drug discovery in Big Pharma: past, present and future. Drug Discov. Today 23, 219–234 (2018).
Camargo, A. C. M., Ianzer, D., Guerreiro, J. R. & Serrano, S. M. T. Bradykinin-potentiating peptides: beyond captopril. Toxicon 59, 516–523 (2012).
Cesa-Luna, C. et al. Structural characterization of scorpion peptides and their bactericidal activity against clinical isolates of multidrug-resistant bacteria. PLoS ONE 14, e0222438 (2019).
Jouiaei, M. et al. Ancient venom systems: a review on Cnidaria toxins. Toxins 7, 2251–2271 (2015).
Jin, A. H. et al. Conotoxins: chemistry and biology. Chem. Rev. 119, 11510–11549 (2019). This review article on conotoxins explains the chemistry and biology behind their function by using 3D structural models, thus providing a deeper understanding of the topic.
McGivern, J. G. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 3, 69–85 (2007).
Safavi-Hemami, H. et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl Acad. Sci. USA 112, 1743–1748 (2015). This article is interesting for researchers involved in peptide hormone research, discussing the weaponization of peptide hormones by animals.
Furman, B. L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 59, 464–471 (2012).
Muller, T. D., Bluher, M., Tschop, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug. Discov. 21, 201–223 (2022).
Rubinstein, E. & Keynan, Y. Vancomycin revisited — 60 years later. Front. Public Health https://doi.org/10.3389/fpubh.2014.00217 (2014).
Heidary, M. et al. Daptomycin. J. Antimicrob. Chemother. 73, 1–11 (2018).
Felnagle, E. A. et al. Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol. Pharm. 5, 191–211 (2008).
Flores, C., Fouquet, G., Moura, I. C., Maciel, T. T. & Hermine, O. Lessons to learn from low-dose cyclosporin-a: a new approach for unexpected clinical applications. Front. Immunol. https://doi.org/10.3389/fimmu.2019.00588 (2019).
Additives, E. et al. Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 15, e05063 (2017).
Nakatsuji, T. & Gallo, R. L. Antimicrobial peptides: old molecules with new ideas. J. Invest. Dermatol. 132, 887–895 (2012).
Lei, J. et al. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 11, 3919 (2012).
Zborovsky, L. et al. Improvement of the antimicrobial potency, pharmacokinetic and pharmacodynamic properties of albicidin by incorporation of nitrogen atoms. Chem. Sci. 12, 14606–14617 (2021). This work is an example of how medicinal chemistry can be used to improve the bioactive qualities of peptides.
Imai, Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464 (2019).
Vilas Boas, L. C. P., Campos, M. L., Berlanda, R. L. A., de Carvalho Neves, N. & Franco, O. L. Antiviral peptides as promising therapeutic drugs. Cell Mol. Life Sci. 76, 3525–3542 (2019).
Bosso, M., Ständker, L., Kirchhoff, F. & Münch, J. Exploiting the human peptidome for novel antimicrobial and anticancer agents. Bioorg. Med. Chem. 26, 2719–2726 (2018).
Kuroki, A., Tay, J., Lee, G. H. & Yang, Y. Y. Broad-spectrum antiviral peptides and polymers. Adv. Healthc. Mater. 10, e2101113 (2021).
Klein, J., Bascands, J.-L., Mischak, H. & Schanstra, J. P. The role of urinary peptidomics in kidney disease research. Kidney Int. 89, 539–545 (2016).
Good, D. M. et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell. Proteom. 9, 2424–2437 (2010).
Argiles, A. et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS ONE 8, e62837 (2013).
Roscioni, S. et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 56, 259–267 (2013).
Nakamura, A. et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018).
Kaya, I., Zetterberg, H., Blennow, K. & Hanrieder, J. R. Shedding light on the molecular pathology of amyloid plaques in transgenic Alzheimer’s disease mice using multimodal MALDI imaging mass spectrometry. ACS Chem. Neurosci. 9, 1802–1817 (2018).
Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 15, 346–366 (2019).
Chen, Z. et al. In-depth site-specific analysis of N-glycoproteome in human cerebrospinal fluid and glycosylation landscape changes in Alzheimer’s disease. Mol. Cell Proteom. 20, 100081 (2021).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Li, Q. et al. Site-specific glycosylation quantitation of 50 serum glycoproteins enhanced by predictive glycopeptidomics for improved disease biomarker discovery. Anal. Chem. 91, 5433–5445 (2019).
Alim, F. Z. D. et al. Seasonal adaptations of the hypothalamo-neurohypophyseal system of the dromedary camel. PLoS ONE 14, e0216679 (2019).
Yu, Q. et al. Targeted mass spectrometry approach enabled discovery of O-glycosylated insulin and related signaling peptides in mouse and human pancreatic islets. Anal. Chem. 89, 9184–9191 (2017).
Anapindi, K. D. B., Romanova, E. V., Checco, J. W. & Sweedler, J. V. Mass spectrometry approaches empowering neuropeptide discovery and therapeutics. Pharmacol. Rev. 74, 662–679 (2022). This review article discusses the historical, current and future states of neuropeptidomics with mass spectrometry and their implications for therapeutic strategies in neurological disorders.
Tillmaand, E. G. et al. Peptidomics and secretomics of the mammalian peripheral sensory-motor system. J. Am. Soc. Mass. Spectrom. 26, 2051–2061 (2015).
Ramachandran, S. et al. A conserved neuropeptide system links head and body motor circuits to enable adaptive behavior. eLife https://doi.org/10.7554/eLife.71747 (2021).
Van Damme, S. et al. Neuromodulatory pathways in learning and memory: lessons from invertebrates. J. Neuroendocrinol. 33, e12911 (2021).
Greenwood, M. P. et al. The effects of aging on biosynthetic processes in the rat hypothalamic osmoregulatory neuroendocrine system. Neurobiol. Aging 65, 178–191 (2018).
Pan, F. et al. Peptidome analysis reveals the involvement of endogenous peptides in mouse pancreatic dysfunction with aging. J. Cell Physiol. 234, 14090–14099 (2019).
Hook, V., Lietz, C. B., Podvin, S., Cajka, T. & Fiehn, O. Diversity of neuropeptide cell–cell signaling molecules generated by proteolytic processing revealed by neuropeptidomics mass spectrometry. J. Am. Soc. Mass. Spectrom. 29, 807–816 (2018).
Anapindi, K. D. B. et al. PACAP and other neuropeptide targets link chronic migraine and opioid-induced hyperalgesia in mouse models. Mol. Cell Proteom. 18, 2447–2458 (2019).
Jiang, Z. et al. Differential neuropeptidomes of dense core secretory vesicles (DCSV) produced at intravesicular and extracellular pH conditions by proteolytic processing. ACS Chem. Neurosci. 12, 2385–2398 (2021).
Podvin, S. et al. Dysregulation of neuropeptide and tau peptide signatures in human Alzheimer’s disease brain. ACS Chem. Neurosci. 13, 1992–2005 (2022).
Al-Hasani, R. et al. In vivo detection of optically-evoked opioid peptide release. eLife https://doi.org/10.7554/eLife.36520 (2018).
Vitorino, R., Guedes, S., Costa, J. P. D. & Kasicka, V. Microfluidics for peptidomics, proteomics, and cell analysis. Nanomaterials https://doi.org/10.3390/nano11051118 (2021).
Ong, T. H., Tillmaand, E. G., Makurath, M., Rubakhin, S. S. & Sweedler, J. V. Mass spectrometry-based characterization of endogenous peptides and metabolites in small volume samples. Biochim. Biophys. Acta 1854, 732–740 (2015).
Burger, T. Gentle introduction to the statistical foundations of false discovery rate in quantitative proteomics. J. Proteome Res. 17, 12–22 (2018). This work is a worthwhile introduction to the statistics behind FDRs, highly recommended for all researchers working in proteomics or peptidomics.
Käll, L., Storey, J. D., MacCoss, M. J. & Noble, W. S. Posterior error probabilities and false discovery rates: two sides of the same coin. J. Proteome Res. 7, 40–44 (2008).
Korthauer, K. et al. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 20, 118 (2019).
Kanz, C. et al. The EMBL nucleotide sequence database. Nucleic Acids Res. 33, D29–D33 (2005).
Fukuda, A., Kodama, Y., Mashima, J., Fujisawa, T. & Ogasawara, O. DDBJ update: streamlining submission and access of human data. Nucleic Acids Res. 49, D71–D75 (2021).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016). This work on the FAIR Guiding Principles is an essential read for all researchers as data management will become more important as data continue to be generated worldwide.
Pichler, K., Warner, K., Magrane, M. & UniProt, C. SPIN: submitting sequences determined at protein level to UniProt. Curr. Protoc. Bioinformatics 62, e52 (2018).
Ternent, T. et al. How to submit MS proteomics data to ProteomeXchange via the PRIDE database. Proteomics 14, 2233–2241 (2014).
Segerstrom, L., Gustavsson, J. & Nylander, I. Minimizing postsampling degradation of peptides by a thermal benchtop tissue stabilization method. Biopreserv. Biobank. 14, 172–179 (2016).
Fridjonsdottir, E., Nilsson, A., Wadensten, H. & Andren, P. E. Brain tissue sample stabilization and extraction strategies for neuropeptidomics. Methods Mol. Biol. 1719, 41–49 (2018).
Stingl, C., Soderquist, M., Karlsson, O., Boren, M. & Luider, T. M. Uncovering effects of ex vivo protease activity during proteomics and peptidomics sample extraction in rat brain tissue by oxygen-18 labeling. J. Proteome Res. 13, 2807–2817 (2014).
Katz, M., Hover, B. M. & Brady, S. F. Culture-independent discovery of natural products from soil metagenomes. J. Ind. Microbiol. Biotechnol. 43, 129–141 (2016).
Reher, R. et al. Native metabolomics identifies the rivulariapeptolide family of protease inhibitors. Nat. Commun. 13, 4619 (2022).
Mills, R. H. et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 7, 262–276 (2022).
Hellinger, R. et al. Peptidomics of circular cysteine-rich plant peptides: analysis of the diversity of cyclotides from viola tricolor by transcriptome and proteome mining. J. Proteome Res. 14, 4851–4862 (2015).
Haynes, W. A., Tomczak, A. & Khatri, P. Gene annotation bias impedes biomedical research. Sci. Rep. 8, 1362 (2018).
Flissi, A. et al. Norine: update of the nonribosomal peptide resource. Nucleic Acids Res. 48, D465–D469 (2020).
Wang, M. et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 34, 828–837 (2016).
Saldivar-Gonzalez, F. I., Aldas-Bulos, V. D., Medina-Franco, J. L. & Plisson, F. Natural product drug discovery in the artificial intelligence era. Chem. Sci. 13, 1526–1546 (2022).
Mohimani, H. et al. Dereplication of peptidic natural products through database search of mass spectra. Nat. Chem. Biol. 13, 30–37 (2017).
Jeanne Dit Fouque, K. et al. Fast and effective ion mobility-mass spectrometry separation of d-amino-acid-containing peptides. Anal. Chem. 89, 11787–11794 (2017).
Hammami, R., Zouhir, A., Le Lay, C., Ben Hamida, J. & Fliss, I. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 10, 22 (2010).
Wang, C. K., Kaas, Q., Chiche, L. & Craik, D. J. CyBase: a database of cyclic protein sequences and structures, with applications in protein discovery and engineering. Nucleic Acids Res. 36, D206–D210 (2008).
Deutsch, E. W. The PeptideAtlas Project. Methods Mol. Biol. 604, 285–296 (2010).
Pineda, S. S. et al. ArachnoServer 3.0: an online resource for automated discovery, analysis and annotation of spider toxins. Bioinformatics 34, 1074–1076 (2018).
wwPDB consortium. Protein Data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Res. 47, D520–D528 (2019).
Larranaga, P. et al. Machine learning in bioinformatics. Brief. Bioinform. 7, 86–112 (2006). This interesting review discusses the machine learning methods that got bioinformatics to where it is today.
Min, S., Lee, B. & Yoon, S. Deep learning in bioinformatics. Brief. Bioinform. 18, 851–869 (2017). This article describes the use, applications and architecture of deep learning networks, providing the readers with insight into the direction that bioinformatics is heading in the next decade.
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Breitling, R. What is systems biology? Front. Physiol. 1, 9 (2010).
Heirendt, L. et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 14, 639–702 (2019).
Mitra, S., Dhar, R. & Sen, R. Designer bacterial cell factories for improved production of commercially valuable non-ribosomal peptides. Biotechnol. Adv. 60, 108023 (2022).
Helmy, M., Smith, D. & Selvarajoo, K. Systems biology approaches integrated with artificial intelligence for optimized metabolic engineering. Metab. Eng. Commun. 11, e00149 (2020).
Acknowledgements
Work in the laboratory of C.W.G. has been supported by the Austrian Science Fund (FWF) through projects P32109 and ZK 81B. The work of A.S. and R.D.S. was funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) under Germany’s Excellence Strategy — EXC 2008-390540038 (UniSysCat) and RTG 2473 ‘Bioactive Peptides’. The work of L.L. is supported by the National Science Foundation (NSF) (CHE-2108223) and National Institutes of Health (NIH) through grants (R01DK071801, R01 AG078794 and RF1AG052324). The work of J.V.S. is supported by the National Institute on Drug Abuse under Award No. P30DA018310 and the National Institute of Neurological Disorders and Stroke (NINDS) through R01NS031609.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Vivian Hook, Dong-Woo Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
antiSMASH: https://antismash.secondarymetabolites.org
DeepBGC: https://github.com/Merck/deepbgc
DeepRiPP: http://deepripp.magarveylab.ca
DEREPLICATOR+: https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp
Dictionary of Natural Products: https://dnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml
High Definition Imaging: https://www.waters.com/waters/en_US/High-Definition-Imaging-(HDI)-Software/nav.htm?cid=134833914&locale=en_US
ImageQuest: https://www.thermofisher.com/order/catalog/product/10137985
MS-FINDER: http://prime.psc.riken.jp/compms/msfinder/main.html
MSiReader: https://msireader.com/
msiQuant: https://ms-imaging.org/paquan/
NCBI: https://www.ncbi.nlm.nih.gov
SANDPUMA: https://bitbucket.org/chevrm/sandpuma/src/master/
SCiLS Lab: https://www.bruker.com/en/products-and-solutions/mass-spectrometry/ms-software/scils-lab.html
UniProt: https://www.uniprot.org
Supplementary information
Glossary
- Fourier transform ion cyclotron resonance mass spectrometers
-
(FTICR-MS). High-resolution mass analysers that trap ions in a cyclotron radius by applying a fixed magnetic field and an oscillating electronic field. As the ions rotate, an interferogram signal is recorded by electrodes and the useful mass spectrum is extracted with a Fourier transformation.
- Hyphenated front-end separation platforms
-
Platforms that separate the analytes online before they enter the mass spectrometers. Techniques include, but are not limited to, liquid chromatography, gas chromatography, ion mobility spectrometry (IMS), solid-phase extraction (SPE) and capillary electrophoresis.
- Ion mobility spectrometry
-
(IMS). An analytical technique that sorts and separates gas-phase ions based on their mobility in a carrier buffer gas under the influence of an electrical field, which is related to the conformation and 3D shapes of the molecules.
- Multiple reaction monitoring
-
A type of analysis for tandem mass spectrometers providing capabilities for quantitation of analytes. Pre-defined precursor ions (m/z) are selected by the first mass analyser and submitted to a fragmentation, and the selected product ion m/z signals are detected by the second mass analyser.
- Peptide dereplication
-
Refers to the identification of known peptides in a sample by comparing mass spectrometric data with a library. The identification can be obtained by comparison of m/z mass signals, including the isotopologue intensities and pattern of isotopologues, giving information on the chemical composition as well as on tandem mass spectrometry (MS/MS) fragmentation spectra match with library data.
- Peptide spectrum match
-
(PSM). A scoring function in which the mass spectrum of a peptide is compared with a theoretical peptide sequence to determine the probability of the measured peptide matching the theoretical peptide.
- Post-source decay
-
A type of fragmentation technique that applies when metastable ions spontaneously decompose in the drift region between the ion source and reflectron.
- Short open reading frames
-
(sORFs). Open reading frames that occur throughout the genome and usually comprise <100 codons. They are a possible source for peptides with biological relevance.
Rights and permissions
About this article
Cite this article
Hellinger, R., Sigurdsson, A., Wu, W. et al. Peptidomics. Nat Rev Methods Primers 3, 25 (2023). https://doi.org/10.1038/s43586-023-00205-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-023-00205-2
This article is cited by
-
Harnessing the power of proteomics in precision diabetes medicine
Diabetologia (2024)