Abstract
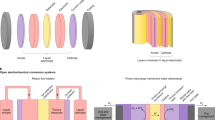
Electrochemistry has the potential to sustainably transform molecules with electrons supplied by renewable electricity. It is one of many solutions towards a more circular, sustainable and equitable society. To achieve this, collaboration between industry and research laboratories is a must. Atomistic understanding from fundamental experiments and modelling can be used to engineer optimized systems whereas limitations set by the scaled-up technology can direct the systems studied in the research laboratory. In this Primer, best practices to run clean laboratory-scale electrochemical systems and tips for the analysis of electrochemical data to improve accuracy and reproducibility are introduced. How characterization and modelling are indispensable in providing routes to garner further insights into atomistic and mechanistic details is discussed. Finally, important considerations regarding material and cell design for scaling up water electrolysis are highlighted and the role of hydrogen in our society’s energy transition is discussed. The future of electrochemistry is bright and major breakthroughs will come with rigour and improvements in the collection, analysis, benchmarking and reporting of electrochemical water splitting data.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Pehl, M. et al. Understanding future emissions from low-carbon power systems by integration of life-cycle assessment and integrated energy modelling. Nat. Energy 2, 939–945 (2017).
Berrang-Ford, L. et al. Tracking global climate change adaptation among governments. Nat. Clim. Chang. 9, 440–449 (2019).
Bogdanov, D. et al. Low-cost renewable electricity as the key driver of the global energy transition towards sustainability. Energy 227, 120467 (2021).
Lèbre, É. et al. The social and environmental complexities of extracting energy transition metals. Nat. Commun. 11, 1–8 (2020).
Akcil, A., Sun, Z. & Panda, S. COVID-19 disruptions to tech-metals supply are a wake-up call. Nature 587, 365–367 (2020).
Herrington, R. Mining our green future. Nat. Rev. Mater. 6, 456–458 (2021).
Bamana, G., Miller, J. D., Young, S. L. & Dunn, J. B. Addressing the social life cycle inventory analysis data gap: Insights from a case study of cobalt mining in the Democratic Republic of the Congo. One Earth 4, 1704–1714 (2021).
Majumdar, A., Deutch, J. M., Prasher, R. S. & Griffin, T. P. A framework for a hydrogen economy. Joule 5, 1905–1908 (2021).
Pingkuo, L. & Xue, H. Comparative analysis on similarities and differences of hydrogen energy development in the world’s top 4 largest economies: a novel framework. Int. J. Hydrog. Energy 47, 9485–9503 (2022).
Crabtree, G. W., Dresselhaus, M. S. & Buchanan, M. V. The hydrogen economy. Phys. Today 57, 39–45 (2004).
Bockris, J. O. A hydrogen economy. Science 176, 1323 (1972).
Ihara, S. Feasibility of hydrogen production by direct water splitting at high temperature. Int. J. Hydrog. Energy 3, 287–296 (1978).
Boettcher, S. W. et al. Potentially confusing: potentials in electrochemistry. ACS Energy Lett. 6, 261–266 (2021).
Hansen, J. N. et al. Is there anything better than Pt for HER? ACS Energy Lett. 6, 1175–1180 (2021).
Kothari, R., Buddhi, D. & Sawhney, R. L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 12, 553–563 (2008).
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015). This work utilizes the protocol from Koper at al. to compare the activity, stability, electrochemically active surface area and Faradic efficiency of ten electrocatalysts for the OER.
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications (Wiley, 2001).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Norskov, J., Bligaard, T., Abild-Pedersen, F. & Studt, F. Fundamental Concepts in Heterogeneous Catalysis (Wiley, 2010).
Tanaka, Y. Ion Exchange Membranes: Fundamentals and Applications (Elsevier Science, 2015).
Luo, T., Abdu, S. & Wessling, M. Selectivity of ion exchange membranes: a review. J. Memb. Sci. 555, 429–454 (2018).
Oener, S. Z., Foster, M. J. & Boettcher, S. W. Accelerating water dissociation in bipolar membranes and for electrocatalysis. Science 369, 1099–1103 (2020).
Tiwari, A., Maagaard, T., Chorkendorff, I. & Horch, S. Effect of dissolved glassware on the structure-sensitive part of the Cu(111) voltammogram in KOH. ACS Energy Lett. 4, 1645–1649 (2019).
Mayrhofer, K. J. J., Wiberg, G. K. H. & Arenz, M. Impact of glass corrosion on the electrocatalysis on Pt electrodes in alkaline electrolyte. J. Electrochem. Soc. 155, P1–P5 (2008).
Mayrhofer, K. J. J., Crampton, A. S., Wiberg, G. K. H. & Arenz, M. Analysis of the impact of individual glass constituents on electrocatalysis on Pt electrodes in alkaline solution. J. Electrochem. Soc. 155, P78–P81 (2008). This work demonstrates the impact of dissolved glassware on the reproducibility of studies using platinum single crystal surfaces in alkaline media.
Fatiadi, A. J. The classical permanganate ion: still a novel oxidant in organic chemistry. Synthesis 1987, 85–127 (1987).
Shaabani, A., Tavasoli-Rad, F. & Lee, D. G. Potassium permanganate oxidation of organic compounds. Synth. Commun. 35, 571–580 (2005).
Arulmozhi, N., Esau, D., van Drunen, J. & Jerkiewicz, G. Design and development of instrumentations for the preparation of platinum single crystals for electrochemistry and electrocatalysis research part 3: final treatment, electrochemical measurements, and recommended laboratory practices. Electrocatalysis 9, 113–123 (2018).
IKA. General guidelines for cleaning electrodes. IKA https://www.ika.com/ika/pdf/flyer-catalog/202103_Electrasyn%202.0_cleaning%20electrodes_EN.pdf (2021).
Kiema, G. K., Aktay, M. & Mcdermott, M. T. Preparation of reproducible glassy carbon electrodes by removal of polishing impurities. J. Electroanal. Chem. 540, 7–15 (2003).
Monteiro, M. C. O. & Koper, M. T. M. Alumina contamination through polishing and its effect on hydrogen evolution on gold electrodes. Electrochim. Acta 325, 134915 (2019). This work demonstrates how contamination introduced during polishing of electrodes affects the activity for the HER.
Raaijman, S. J., Arulmozhi, N., Silva, A. H. M. & Koper, M. T. M. Clean and reproducible voltammetry of copper single crystals with prominent facet-specific features using induction annealing. J. Electrochem. Soc. 168, 096510 (2021).
Kibler, L. A. Preparation and characterization of noble metal single crystal electrode surfaces (International Society of Electrochemistry, 2003). This paper presents a comprehensive guide on the preparation and characterization of noble metal single crystal surfaces for fundamental electrocatalysis studies.
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 355, 6321 (2017).
Shinozaki, K., Zack, J. W., Pylypenko, S., Pivovar, B. S. & Kocha, S. S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique II. Influence of ink formulation, catalyst layer uniformity and thickness. J. Electrochem. Soc. 162, F1384–F1396 (2015).
Morales, D. M., Villalobos, J., Kazakova, M. A., Xiao, J. & Risch, M. Nafion-Induced reduction of manganese and its impact on the electrocatalytic properties of a highly active MnFeNi oxide for bifunctional oxygen conversion. ChemElectroChem 8, 2979–2983 (2021).
Jervis, R. et al. The importance of using alkaline ionomer binders for screening electrocatalysts in alkaline electrolyte. J. Electrochem. Soc. 164, F1551–F1555 (2017). This paper presents guidelines for appropriate ionomer binder choice for screening catalysts in alkaline media.
Birdja, Y. Y. et al. Effects of substrate and polymer encapsulation on CO2 electroreduction by immobilized indium(III) protoporphyrin. ACS Catal. 8, 4420–4428 (2018).
Garsany, Y., Ge, J., St-Pierre, J., Rocheleau, R. & Swider-Lyons, K. E. Analytical procedure for accurate comparison of rotating disk electrode results for the oxygen reduction activity of Pt/C. J. Electrochem. Soc. 161, F628–F640 (2014).
Jerkiewicz, G. Applicability of platinum as a counter-electrode material in electrocatalysis research. ACS Catal. 12, 2661–2670 (2022).
Chen, R. et al. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2, 1070–1075 (2017).
Lee, J. & Bang, J. H. Reliable counter electrodes for the hydrogen evolution reaction in acidic media. ACS Energy Lett. 5, 2706–2710 (2020).
Bird, M. A., Goodwin, S. E. & Walsh, D. A. Best practice for evaluating electrocatalysts for hydrogen economy. ACS Appl. Mater. Interfaces 12, 20500–20506 (2020). This work recommends carbon counter electrodes separated from the working electrode compartment by a frit to avoid cross-contamination in studies of the HER.
Ji, S. G., Kim, H., Park, C., Kim, W. & Choi, C. H. Underestimation of platinum electrocatalysis induced by carbon monoxide evolved from graphite counter electrodes. ACS Catal. 10, 10773–10783 (2020).
Jerkiewicz, G. Standard and reversible hydrogen electrodes: theory, design, operation, and applications. ACS Catal. 10, 8409–8417 (2020).
Nu, S., Li, S., Du, Y., Han, X. & Xu, P. How to reliably report the overpotential of an electrocatalyst. ACS Energy Lett. 5, 1083–1087 (2020).
Leung, K. Y. & Mccrory, C. C. L. Effect and prevention of trace Ag+ contamination from Ag/AgCl reference electrodes on CO2 reduction product distributions at polycrystalline copper electrodes. ACS Appl. Energy Mater. 2, 8283–8293 (2019).
Roger, I. & Symes, M. D. Silver leakage from Ag/AgCl reference electrodes as a potential cause of interference in the electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces 9, 472–478 (2017).
Mousavi, M. P. S., Saba, S. A., Anderson, E. L., Hillmyer, M. A. & Bühlmann, P. Avoiding errors in electrochemical measurements: effect of frit material on the performance of reference electrodes with porous frit junctions. Anal. Chem. 88, 8706–8713 (2016).
Zeledón, J. A. Z., Jackson, A., Stevens, M. B., Kamat, G. A. & Jaramillo, T. F. Methods — a practical approach to the reversible hydrogen electrode scale. J. Electrochem. Soc. 169, 066505 (2022).
Rosca, V., Duca, M., de Groot, M. T. & Koper, M. T. M. Nitrogen cycle electrocatalysis. Chem. Rev. 109, 2209–2244 (2009).
Subbaraman, R. et al. Origin of anomalous activities for electrocatalysts in alkaline electrolytes. J. Phys. Chem. C. 116, 22231–22237 (2012).
Kodama, K., Jinnouchi, R., Takahashi, N., Murata, H. & Morimoto, Y. Activities and stabilities of Au-modified stepped-Pt single-crystal electrodes as model cathode catalysts in polymer electrolyte fuel cells. J. Am. Chem. Soc. 138, 4194–4200 (2016).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Corrigan, D. A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 134, 377–384 (1987).
Ojha, K., Doblhoff-Dier, K. & Koper, M. T. M. Double-layer structure of the Pt(111)–aqueous electrolyte interface. Proc. Natl Acad. Sci. USA 119, e2116016119 (2022).
Sheberla, D. et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–225 (2017).
Wuttig, A. & Surendranath, Y. Impurity ion complexation enhances carbon dioxide reduction catalysis. ACS Catal. 5, 4479–4484 (2015).
Spanos, I. et al. Facile protocol for alkaline electrolyte purification and its influence on a Ni–Co oxide catalyst for the oxygen evolution reaction. ACS Catal. 9, 8165–8170 (2019).
Liu, L. et al. Purification of residual Ni and Co hydroxides from Fe-free alkaline electrolyte for electrocatalysis studies. ChemElectroChem https://doi.org/10.1002/celc.202200279 (2022).
Rebollar, L., Intikhab, S., Snyder, J. D. & Tang, M. H. Kinetic isotope effects quantify pH-sensitive water dynamics at the Pt electrode interface. J. Phys. Chem. L 11, 2308–2313 (2020).
Anderson, C. E. & Ebenhaek, D. G. in Analysis of Essential Nuclear Reactor Materials (ed. Rodden, C. J.) 629–661 (US Atomic Energy Commission, 1964).
Schmidt, T. J. et al. Characterization of high-surface-area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 145, 2354–2358 (1998).
Levich, V. G. in Physicochemical Hydrodynamics 60–72 (Prentice-Hall Englewood, 1962).
Villullas, H. M. & Lopez Teijelo, M. Meniscus shape and lateral wetting at the hanging meniscus rotating disc (HMRD) electrode. J. Appl. Electrochem. 26, 353–359 (1996).
Janssen, L. J. J., Sillen, C. W. M. P., Barendrecht, E. & van Stralen, S. J. D. Bubble behaviour during oxygen and hydrogen evolution at transparent electodes in KOH solution. Electrochim. Acta 29, 633–642 (1984).
Matsuura, K., Yamanishi, Y., Guan, C. & Yanase, S. Control of hydrogen bubble plume during electrolysis of water. J. Phys. Commun. 3, 035012 (2019).
Hodges, A. et al. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 13, 1304 (2022).
Zhao, X., Ren, H. & Luo, L. Gas bubbles in electrochemical gas evolution reactions. Langmuir 35, 5392–5408 (2019).
Angulo, A., Linde, P., van der, Gardeniers, H., Modestino, M. & Rivas, D. F. Influence of bubbles on the energy conversion efficiency of electrochemical reactors. Joule 4, 555–579 (2020). This work demonstrates how bubbles impact the energy efficiency of electrocatalytic processes.
Zdunek, A. D. & Selman, J. R. A novel rotating disk electrode cell design: the inverted rotating disk. J. Electrochem. Soc. 139, 2549–2551 (1992).
Meethal, R. P., Saibi, R. & Srinivasan, R. Hydrogen evolution reaction on polycrystalline Au inverted rotating disc electrode in HClO4 and NaOH solutions. Int. J. Hydrog. Energy 47, 14304–14318 (2022).
Vos, J. G. & Koper, M. T. M. M. Examination and prevention of ring collection failure during gas-evolving reactions on a rotating ring-disk electrode. J. Electroanal. Chem. 850, 113363 (2019).
Shih, A. J., Arulmozhi, N. & Koper, M. T. M. Electrocatalysis under cover: enhanced hydrogen evolution via defective graphene-covered Pt(111). ACS Catal. 11, 10892–10901 (2021).
Hsu, J. P. Recommended pre-operation cleanup procedures for hydrogen fueling station. Int. J. Hydrog. Energy 37, 1770–1780 (2011).
Wan, C. T. et al. A potential-dependent Thiele modulus to quantify the effectiveness of porous electrocatalysts. Preprint at https://doi.org/10.26434/chemrxiv-2021-cwqm0-v2 (2021). This work demonstrates the coupling of diffusion reaction-governing equations with macroscopic catalytic rates to quantify the extent of internal mass transfer limitations.
Hickman, D. A., Degenstein, J. C. & Ribeiro, F. H. Fundamental principles of laboratory fixed bed reactor design. Curr. Opin. Chem. Eng. 13, 1–9 (2016).
Harris, J. W. et al. Consequences of product inhibition in the quantification of kinetic parameters. J. Catal. 389, 468–475 (2020).
Kamat, G. A. et al. Acid anion electrolyte effects on platinum for oxygen and hydrogen electrocatalysis. Commun. Chem. 5, 1–10 (2022).
Zhang, Y., Zhang, H., Ji, H., Chen, C. & Zhao, J. Pivotal role and regulation of proton transfer in water oxidation on hematite photoanodes. J. Am. Chem. Soc. 138, 2705–2711 (2016).
Yang, H. et al. Intramolecular hydroxyl nucleophilic attack pathway by a polymeric water oxidation catalyst with single cobalt sites. Nat. Catal. 5, 414–429 (2022).
Chen, Z. et al. Concerted O atom-proton transfer in the O–O bond forming step in water oxidation. Proc. Natl Acad. Sci. USA 107, 7225–7229 (2010).
Xia, C. et al. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide. Nat. Catal. 3, 125–134 (2020).
Rabe, M. et al. Alkaline manganese electrochemistry studied by in situ and operando spectroscopic methods — metal dissolution, oxide formation and oxygen evolution. Phys. Chem. Chem. Phys. 21, 10457–10469 (2019).
Huang, J. et al. In situ monitoring of the electrochemically induced phase transition of thermodynamically metastable 1T-MoS2 at nanoscale. Nanoscale 12, 9246–9254 (2020).
Shpigel, N., Levi, M. D., Sigalov, S., Daikhin, L. & Aurbach, D. In situ real-time mechanical and morphological characterization of electrodes for electrochemical energy storage and conversion by electrochemical quartz crystal microbalance with dissipation monitoring. Acc. Chem. Res. 51, 69–79 (2018).
Hodnik, N., Dehm, G. & Mayrhofer, K. J. J. Importance and challenges of electrochemical in situ liquid cell electron microscopy for energy conversion research. Acc. Chem. Res. 49, 2015–2022 (2016). This work applies in situ liquid cell electron microscopy for electrocatalysis research.
Velasco-Velez, J.-J. et al. Revealing the active phase of copper during the electroreduction of CO2 in aqueous electrolyte by correlating in situ X-ray spectroscopy and in situ electron microscopy. ACS Energy Lett. 5, 2106–2111 (2020).
Zhang, L., Shi, W. & Zhang, B. A review of electrocatalyst characterization by transmission electron microscopy. J. Energy Chem. 26, 1117–1135 (2017).
Timoshenko, J. & Roldan Cuenya, B. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 121, 882–961 (2021). This work applies in situ and operando XAS to probe the interactions of working electrocatalysts with the environment and its structural, chemical and electronic transformations.
Velasco-Velez, J.-J. et al. A comparative study of electrochemical cells for in situ X-ray spectroscopies in the soft and tender X-ray range. J. Phys. D. Appl. Phys. 54, 124003 (2021).
Liu, Y. et al. Transition metal nitrides as promising catalyst supports for tuning CO/H2 syngas production from electrochemical CO2 reduction. Angew. Chem. Int. Ed. 59, 11345–11348 (2020).
Sasaki, K., Marinkovic, N., Isaacs, H. S. & Adzic, R. R. Synchrotron-based in situ characterization of carbon-supported platinum and platinum monolayer electrocatalysts. ACS Catal. 6, 69–76 (2016).
Sugawara, Y., Yadav, A. P., Nishikata, A. & Tsuru, T. Electrochemical quartz crystal microbalance study on dissolution of platinum in acid solutions. Electrochemistry 75, 359–365 (2007).
Levi, M. D., Salitra, G., Levy, N., Aurbach, D. & Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nat. Mater. 8, 872–875 (2009).
Wang, Y.-H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021). This research paper provides modelling guidelines to assess the structure of the electrochemical interface including water, cations and electric field. Theoretical results are benchmarked through in situ Raman spectroscopy characterization.
Dong, J.-C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Liang, Y. et al. Electrochemical scanning probe microscopies in electrocatalysis. Small Methods 3, 1800387 (2019).
Simon, G. H., Kley, C. S. & Roldan Cuenya, B. Potential-dependent morphology of copper catalysts during CO2 electroreduction revealed by in situ atomic force microscopy. Angew. Chem. Int. Ed. 60, 2561–2568 (2021).
Li, J. F., Zhang, Y. J., Ding, S. Y., Panneerselvam, R. & Tian, Z. Q. Core-shell nanoparticle-enhanced raman spectroscopy. Chem. Rev. 117, 5002–5069 (2017).
Kas, R., Ayemoba, O., Firet, N. J., Middelkoop, J. & Smith, W. A. In-situ infrared spectroscopy applied to the study of the electrocatalytic reduction of CO2: theory, practice and challenges. ChemPhysChem 20, 2904–2925 (2019).
Zhu, S., Li, T., Cai, W.-B. & Shao, M. CO2 electrochemical reduction as probed through infrared spectroscopy. ACS Energy Lett. 4, 682–689 (2019).
Zhang, Z.-Q., Banerjee, S., Thoi, V. S. & Shoji Hall, A. Reorganization of interfacial water by an amphiphilic cationic surfactant promotes CO2 reduction. J. Phys. Chem. Lett. 11, 5457–5463 (2020).
Friebel, D. et al. Balance of nanostructure and bimetallic interactions in Pt model fuel cell catalysts: in situ XAS and DFT study. J. Am. Chem. Soc. 134, 9664–9671 (2012).
Wu, C. H. et al. The structure of interfacial water on gold electrodes studied by X-ray absorption spectroscopy. Science 346, 831–834 (2014).
Mom, R. et al. The oxidation of platinum under wet conditions observed by electrochemical X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 141, 6537–6544 (2019).
Velasco-Velez, J. J. et al. Photoelectron spectroscopy at the graphene–liquid interface reveals the electronic structure of an electrodeposited cobalt/graphene electrocatalyst. Angew. Chem. Int. Ed. 54, 14554–14558 (2015).
Favaro, M. et al. An operando investigation of (Ni–Fe–Co–Ce)Ox system as highly efficient electrocatalyst for oxygen evolution reaction. ACS Catal. 7, 1248–1258 (2017).
Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2, 17031 (2017).
Favaro, M. et al. Unravelling the electrochemical double layer by direct probing of the solid/liquid interface. Nat. Commun. 7, 1–8 (2016).
Rao, R. R. et al. Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516–525 (2020).
Reikowski, F. et al. Operando surface X-ray diffraction studies of structurally defined Co3O4 and CoOOH thin films during oxygen evolution. ACS Catal. 9, 3811–3821 (2019).
Gründer, Y. & Lucas, C. A. Surface X-ray diffraction studies of single crystal electrocatalysts. Nano Energy 29, 378–393 (2016).
Bogar, M. et al. Interplay among dealloying, ostwald ripening, and coalescence in PtXNi100–X bimetallic alloys under fuel-cell-related conditions. ACS Catal. 11, 11360–11370 (2021).
Jacobse, L., Rost, M. J. & Koper, M. T. M. Atomic-scale identification of the electrochemical roughening of platinum. ACS Cent. Sci. 5, 1920–1928 (2019).
Wang, X. et al. In situ scanning tunneling microscopy of cobalt-phthalocyanine-catalyzed CO2 reduction reaction. Angew. Chem. 59, 16098–16103 (2020).
Wang, X. et al. Mechanistic reaction pathways of enhanced ethylene yields during electroreduction of CO2–CO co-feeds on Cu and Cu-tandem electrocatalysts. Nat. Nanotechnol. 14, 1063–1070 (2019).
Davies, B. J. V., Arenz, M., Rossmeisl, J. & Escudero-Escribano, M. Electrochemical synthesis of high-value chemicals: detection of key reaction intermediates and products combining gas chromatography−mass spectrometry and in situ infrared spectroscopy. J. Phys. Chem. C. 123, 12762–12772 (2019).
Kwon, Y. & Koper, M. T. M. Combining voltammetry with HPLC: application to electro-oxidation of glycerol. Anal. Chem. 82, 5420–5424 (2010).
Zeng, R. et al. Methanol oxidation using ternary ordered intermetallic electrocatalysts: a DEMS study. ACS Catal. 10, 770–776 (2020).
Stoerzinger, K. A. et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2, 876–881 (2017).
Todoroki, N., Tsurumaki, H., Tei, H., Mochizuki, T. & Wadayama, T. Online electrochemical mass spectrometry combined with the rotating disk electrode method for direct observations of potential-dependent molecular behaviors in the electrode surface vicinity. J. Electrochem. Soc. 167, 106503 (2020).
Geiger, S. et al. The stability number as a metric for electrocatalyst stability benchmarking. Nat. Catal. 1, 508–515 (2018).
Lopes, P. P. et al. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 6, 2536–2544 (2016).
Kasian, O. et al. Degradation of iridium oxides via oxygen evolution from the lattice: correlating atomic scale structure with reaction mechanisms. Energy Environ. Sci. 12, 3548–3555 (2019).
Klemm, S. O., Topalov, A. A., Laska, C. A. & Mayrhofer, K. J. J. Coupling of a high throughput microelectrochemical cell with online multielemental trace analysis by ICP-MS. Electrochem. Commun. 13, 1533–1535 (2011).
Kunimatsu, K., Senzaki, T., Samejeske, G., Tsushima, M. & Osawa, M. Hydrogen adsorption and hydrogen evolution reaction on a polycrystalline Pt electrode studied by surface-enhanced infrared absorption spectroscopy. Electrochim. Acta 52, 5715–5724 (2007).
Scott, S. B. et al. The low overpotential regime of acidic water oxidation part I: the importance of O2 detection. Energy Environ. Sci. https://doi.org/10.1039/d1ee03914h (2022).
Yokoyama, Y. et al. In situ local pH measurements with hydrated iridium oxide ring electrodes in neutral pH aqueous solutions. Chem. Lett. 49, 195–198 (2020).
Monteiro, M. C. O., Liu, X., Hagedoorn, B. J. L., Snabilié, D. D. & Koper, M. T. M. Interfacial pH measurements using a rotating ring-disc electrode with a voltammetric pH sensor. ChemElectroChem 9, e202101223 (2022).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Čolić, V. et al. Experimental aspects in benchmarking of the electrocatalytic activity. ChemElectroChem 2, 143–149 (2015).
Zheng, J., Yan, Y. & Xu, B. Correcting the hydrogen diffusion limitation in rotating disk electrode measurements of hydrogen evolution reaction kinetics. J. Electrochem. Soc. 162, F1470–F1481 (2015).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31, 1806296 (2019). This work expands our coverage on the collection and analysis of reaction rates, kinetics and normalization of catalyst activity.
Voiry, D. et al. Best practices for reporting electrocatalytic performance of nanomaterials. ACS Nano 12, 9635–9638 (2018).
Prats, H. & Chan, K. The determination of the HOR/HER reaction mechanism from experimental kinetic data. Phys. Chem. Chem. Phys. https://doi.org/10.1039/d1cp04134g (2021).
Wei, C., Sun, S., Mandler, D. & Wang, X. Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity. Chem Soc Rev. https://doi.org/10.1039/c8cs00848e (2019).
Garreau, D. & Saveant, J. M. Linear sweep voltammetry — compensation of cell resistance and stability. Electroanal. Chem. Interfacial Electrochem. 35, 309–331 (1972).
Koutecky, J. & Levich, V. G. The application of the rotating disc electrode to studies of kinetic and catalytic processes. Zh. Fiz. Khim. 32, 1565–1575 (1958).
Fogler, H. S. Essentials of Chemical Reaction Engineering (Pearson Education, 2010).
Monteiro, M. C. O., Jacobse, L., Touzalin, T. & Koper, M. T. M. Mediator-free SECM for probing the diffusion layer pH with functionalized gold ultramicroelectrodes. Anal. Chem. 92, 2237–2243 (2020).
Monteiro, M. C. O. & Koper, M. T. M. Measuring local pH in electrochemistry. Curr. Opin. Electrochem. 25, 100649 (2021).
Goyal, A. & Koper, M. T. M. Understanding the role of mass transport in tuning the hydrogen evolution kinetics on gold in alkaline media. J. Chem. Phys. 155, 134705 (2021).
Hasan, M. H. & McCrum, I. T. Understanding the role of near-surface solvent in electrochemical adsorption and electrocatalysis with theory and experiment. Curr. Opin. Electrochem. 33, 100937 (2022).
Taylor, H. S. The mechanism of activation at catalytic surfaces. Proc. R. Soc. A Math. Phys. Eng. Sci. 113, 77–86 (1926).
Boudart, M. Turnover rates in heterogeneous catalysis. Chem. Rev. 95, 661–666 (1995).
Kozuch, S. & Martin, J. M. L. “Turning Over” definitions in catalytic cycles. ACS Catal. 2, 2787–2794 (2012).
Anantharaj, S., Karthik, P. E. & Noda, S. The significance of properly reporting turnover frequency in electrocatalysis research. Angew. Chem. Int. Ed. 60, 23051–23067 (2021).
Barber, J., Morin, S. & Conway, B. E. Specificity of the kinetics of H2 evolution to the structure of single-crystal Pt surfaces, and the relation between OPD and UPD H. J. Electroanal. Chem. 446, 125–138 (1998).
Weber, R. S. Lies, damned lies, and turnover rates. J. Catal. 404, 925–928 (2021).
Trasatti, S. & Petrii, O. A. Real surface area measurements in electrochemistry. J. Electroanal. Chem. 327, 353–376 (1993).
Li, D., Batchelor-McAuley, C. & Compton, R. G. Some thoughts about reporting the electrocatalytic performance of nanomaterials. Appl. Mater. Today 18, 100404 (2020).
Hou, S. et al. A review on experimental identification of active sites in model bifunctional electrocatalytic systems for oxygen reduction and evolution reactions. ChemElectroChem 8, 3433–3456 (2021).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–103 (2007).
Anantharaj, S. & Kundu, S. Do the evaluation parameters reflect intrinsic activity of electrocatalysts in electrochemical water splitting? ACS Energy Lett. 4, 1260–1264 (2019).
Stoerzinger, K. A., Qiao, L., Biegalski, M. D. & Shao-Horn, Y. Orientation-dependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 5, 1636–1641 (2014).
Kluge, R. M., Haid, R. W. & Bandarenka, A. S. Assessment of active areas for the oxygen evolution reaction on an amorphous iridium oxide surface. J. Catal. 396, 14–22 (2021).
Aufa, M. H. et al. Fast and accurate determination of the electroactive surface area of MnOx. Electrochim. Acta 389, 138692 (2021).
Watzele, S. & Bandarenka, A. S. Quick determination of electroactive surface area of some oxide electrode materials. Electroanalysis 28, 2394–2399 (2016).
Watzele, S. et al. Determination of electroactive surface area of Ni-, Co-, Fe-, and Ir-based oxide electrocatalysts. ACS Catal. 9, 9222–9230 (2019).
Quast, T. et al. Single particle nanoelectrochemistry reveals the catalytic oxygen evolution reaction activity of Co3O4 nanocubes. Angew. Chem. Int. Ed. 60, 23444–23450 (2021).
Shinagawa, T., Garcia-Esparza, A. T. & Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 5, 13801 (2015). This work utilizes microkinetic modelling to demonstrate how different rate-limiting steps and surface coverages impact Tafel slopes and reaction orders.
Jung, O., Jackson, M. N., Bisbey, R. P., Kogan, N. E. & Surendranath, Y. Innocent buffers reveal the intrinsic pH- and coverage-dependent kinetics of the hydrogen evolution reaction on noble metals. Joule 6, 476–493 (2022).
Mitchell, J. B., Shen, M., Twight, L. & Boettcher, S. W. Hydrogen-evolution-reaction kinetics pH dependence: is it covered? Chem. Catal https://doi.org/10.1016/j.checat.2022.02.001 (2022).
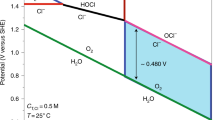
Vos, J. G., Venugopal, A., Smith, W. A. & Koper, M. T. M. Competition and selectivity during parallel evolution of bromine, chlorine and oxygen on IrOx electrodes. J. Catal. 389, 99–110 (2020).
Satterfield, C. N. Mass Transfer in Heterogeneous Catalysis (MIT Press, 1970).
Monteiro, M. C. O., Goyal, A., Moerland, P. & Koper, M. T. M. Understanding cation trends for hydrogen evolution on platinum and gold electrodes in alkaline media. ACS Catal. 11, 14328–14335 (2021).
Chen, Q., Solla-gullón, J., Sun, S. & Feliu, J. M. The potential of zero total charge of Pt nanoparticles and polycrystalline electrodes with different surface structure: the role of anion adsorption in fundamental electrocatalysis. Electrochim. Acta 55, 7982–7994 (2010).
Grgur, B. N., Marković, N. M. & Ross, P. N. Temperature-dependent oxygen electrochemistry on platinum low-index single crystal surfaces in acid solutions. Can. J. Chem. 75, 1465–1471 (1997).
Gojković, S. L., Zečević, S. K. & Dražić, D. M. Oxygen reduction on iron. Part VII. Temperature dependence of oxygen reduction on passivated iron in alkaline solutions. J. Electroanal. Chem. 399, 127–133 (1995).
Tang, Z. Q., Liao, L. W., Zheng, Y. L., Kang, J. & Chen, Y. X. Temperature effect on hydrogen evolution reaction at Au electrode. Chin. J. Chem. Phys. 25, 469–474 (2012).
Kang, J., Lin, C. H., Yao, Y. & Chen, Y. X. Kinetic implication from temperature effect on hydrogen evolution reaction at Ag electrode. Chin. J. Chem. Phys. 27, 63–68 (2014).
Watzele, S. A., Katzenmeier, L., Sabawa, J. P., Garlyyev, B. & Bandarenka, A. S. Electrochimica acta temperature dependences of the double layer capacitance of some solid/liquid and solid/solid electrified interfaces. An experimental study. Electrochim. Acta 391, 138969 (2021).
Garcia-Araez, N., Climent, V. & Feliu, J. M. Temperature effects on platinum single-crystal electrodes. Russ. J. Electrochem. 48, 271–280 (2012).
Marković, N. M. et al. Effect of temperature on surface processes at the Pt(111)–liquid interface: hydrogen adsorption, oxide formation, and CO oxidation. J. Phys. Chem. B 103, 8568–8577 (1999).
Gomez, R., Orts, J. M., Alvarez-Ruiz, B. & Feliu, J. M. Effect of temperature on hydrogen adsorption on Pt(111), Pt(110), and Pt(100) electrodes. J. Phys. Chem. 108, 228–238 (2004).
He, Z. D., Chen, Y. X., Santos, E. & Schmickler, W. The pre-exponential factor in electrochemistry. Angew. Chem.- Int. Ed. 57, 7948–7956 (2018).
Vos, R. E. & Koper, M. T. M. The effect of temperature on the cation-promoted electrochemical CO2 reduction on gold. ChemElectroChem 9, e202200239 (2022).
Conway, B. E. & Wilkinson, D. P. Non-isothermal cell potentialas and evaluation of entropies of ions and of activation for single electrode processes in non-aqueous media. Electrochim. Acta 38, 997–1013 (1993).
Conway, B. E. & Wilkinsont, D. P. Comparison of entropic and enthalpic components of the barrier symmetry factor, β, for proton discharge at liquid and solid Hg in relation to the variation of Tafel slopes and β with temperature. J. Chem. Soc. Faraday Trans. 85, 2355–2367 (1989).
Wildgoose, G. G., Giovanelli, D., Lawrence, N. S. & Compton, R. G. High-temperature electrochemistry: a review. Electroanalysis 16, 421–433 (2004).
Uwitonze, N., Chen, W., Zhou, D., He, Z. & Chen, Y.-X. The determination of thermal junction potential difference. Sci. China Chem. 61, 1020–1024 (2018).
Öijerholm, J., Forsberg, S., Hermansson, H.-P. & Ullberg, M. Relation between the SHE and the internal Ag/AgCl reference electrode at high temperatures. J. Electrochem. Soc. 156, P56–P61 (2009).
National Institute of Standards and Technology. IUPAC-NIST solubility database, version 1.1. NIST standard reference database 106. SRDTA https://doi.org/10.18434/T4QC79 (2012).
Williams, K., Limaye, A., Weiss, T., Chung, M. & Manthiram, K. Accounting for species’ thermodynamic activities changes mechanistic interpretations of electrochemical kinetic data. Preprint at https://doi.org/10.26434/chemrxiv-2022-vk5z9 (2022). This work demonstrates how accounting for the thermodynamic activities of species can impact reaction order measurements, potentially leading to faulty mechanistic interpretations.
Haschke, S. et al. Direct oxygen isotope effect identifies the rate-determining step of electrocatalytic OER at an oxidic surface. Nat. Commun. https://doi.org/10.1038/s41467-018-07031-1 (2018).
Pasquini, C. et al. H/D isotope effects reveal factors controlling catalytic activity in Co-based oxides for water oxidation. J. Am. Chem. Soc. 141, 2938–2948 (2019).
Tse, E. C. M., Hoang, T. T. H., Varnell, J. A. & Gewirth, A. A. Observation of an inverse kinetic isotope effect in oxygen evolution electrochemistry. ACS Catal. 6, 5706–5714 (2016).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005).
McCrum, I. T. & Koper, M. T. M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 5, 891–899 (2020). This research paper introduces *OH adsorption strength as an additional descriptor for HER activity in addition to *H binding, and also provides guidelines for assessing the kinetics of the HER in alkaline media and in the presence of cations.
Koper, M. T. M. Thermodynamic theory of multi-electron transfer reactions: implications for electrocatalysis. J. Electroanal. Chem. 660, 254–260 (2011).
Katayama, Y. et al. An in situ surface-enhanced infrared absorption spectroscopy study of electrochemical CO2 reduction: selectivity dependence on surface C-bound and O-bound reaction intermediates. J. Phys. Chem. C. 123, 5951–5963 (2019).
Feibelman, P. J. Surface-diffusion mechanism versus electric field: Pt/Pt(001). Phys. Rev. B Condens. Matter Mater. Phys. 64, 125403 (2001).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 1–7 (2009).
Henkelman, G. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Sanville, E., Kenny, S. D., Smith, R. & Henkelman, G. Improved grid-based algorithm for bader charge allocation. J. Comput. Chem. 28, 899–908 (2007).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Monteiro, M. C. O., Dattila, F., Lopez, N. & Koper, M. T. M. The role of cation acidity on the competition between hydrogen evolution and CO2 reduction on gold electrodes. J. Am. Chem. Soc. 144, 1589–1602 (2022).
Gupta, N., Gattrell, M. & Macdougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Elecrochem. 36, 161–172 (2006).
Weng, L.-C., Bell, A. T. & Weber, A. Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018).
Cheng, D. et al. The nature of active sites for carbon dioxide electroreduction over oxide-supported copper catalysts. Nat. Commun. 12, 395 (2021).
Pérez-Ramírez, J. & López, N. Strategies to break linear scaling relationships. Nat. Catal. 2, 971–976 (2019).
Rossmeisl, J., Logadottir, A. & Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 319, 178–184 (2005).
Rossmeisl, J., Qu, Z.-W., Zhu, H., Kroes, G.-J. & Norskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Brönsted, J. N. Acid and basic catalysis. Chem. Rev. 5, 231–338 (1928).
Evans, M. G. & Polanyi, M. Inertia and driving force of chemical reaction. Trans. Faraday Soc. 34, 11–24 (1938).
Bligaard, T. et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Goyal, A. & Koper, M. T. M. The interrelated effect of cations and electrolyte pH on the hydrogen evolution reaction on gold electrodes in alkaline media. Angew. Chem. Int. Ed. 60, 13452–13462 (2021).
Ong, S. P. et al. Python Materials Genomics (pymatgen): a robust, open-source Python library for materials analysis. Comput. Mater. Sci. 68, 314–319 (2013).
Gunasooriya, G. T. K. K., Nørskov, J. K. Analysis of acid-stable and active oxides for the oxygen evolution reaction. ACS Energy Lett. 5, 3778–3787 (2020).
Zhou, L. et al. Rutile alloys in the Mn−Sb−O system stabilize Mn3+ to enable oxygen evolution in strong acid. ACS Catal. 8, 10938–10948 (2018).
Gunasooriya, G. T. K. K. et al. First-row transition metal antimonates for the oxygen reduction reaction. ACS Nano 16, 6334–6348 (2022).
Kastlunger, G., Lindgren, P. & Peterson, A. A. Controlled-potential simulation of elementary electrochemical reactions: proton discharge on metal surfaces. J. Phys. Chem. C. 122, 12771–12781 (2018).
Warburton, R. E., Soudackov, A. V. & Hammes-Schiffr, S. Theoretical modeling of electrochemical proton-coupled electron transfer. Chem. Rev. https://doi.org/10.1021/acs.chemrev.1c00929 (2022).
Abidi, N., Lim, K. R. G., Seh, Z. W. & Steinmann, S. N. Atomistic modeling of electrocatalysis: are we there yet? WIREs Comput. Mol. Sci. 11, e1499 (2021). This outstanding review paper offers a detailed discussion on the different methodologies developed to model the electrochemical interface, including the computational hydrogen electrode, constant potential and constant electric field approaches (as summarized in the paper in Fig. 3).
Sundararaman, R., Vigil-fowler, D. & Schwarz, K. Improving the accuracy of atomistic simulations of the electrochemical interface. Chem. Rev. 122, 10651–10674 (2022).
Groß, A. & Sakong, S. Ab initio simulations of water/metal interfaces. Chem. Rev. 122, 10746–10776 (2022).
Pohlmann, S. Metrics and methods for moving from research to innovation in energy storage. Nat. Commun. 13, 1538 (2022).
Zheng, Y., Jiao, Y., Vasileff, A. & Qiao, S. Z. The hydrogen evolution reaction in alkaline solution: from theory, single crystal models, to practical electrocatalysts. Angew. Chem. Int. Ed. 57, 7568–7579 (2018).
Millet, P. & Grigoriev, S. in Renewable Hydrogen Technologies (eds Gandia, L. M., Arzamendi, G. & Dieguez, P. M.) 19–41 (Elsevier, 2013). This text presents an overview of mature and laboratory-scale water electrolysis technologies, and provides some common values of important process parameters, which helps put the process requirements in perspective.
Burton, N. A., Padilla, R. V., Rose, A. & Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 135, 110255 (2021).
Wang, M., Wang, Z., Gong, X. & Guo, Z. The intensification technologies to water electrolysis for hydrogen production — a review. Renew. Sustain. Energy Rev. 29, 573–588 (2014).
Barati, G., Aliofkhazraei, M. & Shanmugam, S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting. Renew. Sustain. Energy Rev. 114, 109300 (2019).
Rodríguez, J. & Amores, E. CFD modeling and experimental validation of an alkaline water electrolysis cell for hydrogen production. Processes 8, 1634 (2020). This work introduces important engineering parameters that need to be taken into account when designing electrochemical cells.
Knöppel, J. et al. On the limitations in assessing stability of oxygen evolution catalysts using aqueous model electrochemical cells. Nat. Commun. 12, 1–9 (2021).
Watzele, S., Liang, Y. & Bandarenka, A. S. Intrinsic activity of some oxygen and hydrogen evolution reaction electrocatalysts under industrially relevant conditions. ACS Appl. Energy Mater. 1, 4196–4202 (2018).
Lazaridis, T., Stühmeier, B. M., Gasteiger, H. A. & El-Sayed, H. A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 5, 363–373 (2022).
Scott, K. Handbook of Industrial Membranes 271–305 (Elsevier, 1995).
Wang, S. et al. Modifying ionic membranes with carbon dots enables direct production of high-purity hydrogen through water electrolysis. ACS Appl. Mater. Interfaces 13, 39304–39310 (2021).
Ligen, Y., Vrubel, H. & Girault, H. Energy efficient hydrogen drying and purification for fuel cell vehicles. Int. J. Hydrog. Energy 45, 10639–10647 (2020).
Akbashev, A. R. Electrocatalysis goes nuts. ACS Catal. 12, 4296–4301 (2022).
Shinozaki, K., Zack, J. W., Richards, R. M., Pivovar, B. S. & Kocha, S. S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique: I. Impact of impurities, measurement protocols and applied corrections. J. Electrochem. Soc. 162, F1144–F1158 (2015).
Christopher, P., Jin, S., Sivula, K. & Kamat, P. V. Why seeing is not always believing: common pitfalls in photocatalysis and electrocatalysis. ACS Energy Lett. 6, 707–709 (2021).
McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013). This work recommends a protocol to reliably compare the activity, stability, electrochemically active surface area and Faradic efficiency of electrocatalysts for the OER.
Chen, J. G., Jones, C. W., Linic, S. & Stamenkovic, V. R. Best practices in pursuit of topics in heterogeneous electrocatalysis. ACS Catal. 7, 6392–6393 (2017).
Bligaard, T. et al. Toward benchmarking in catalysis science: best practices, challenges, and opportunities. ACS Catal. 6, 2590–2602 (2016).
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Kocha, S. S. et al. Best practices and testing protocols for benchmarking ORR activities of fuel cell electrocatalysts using rotating disk electrode. Electrocatalysis 8, 366–374 (2017).
Anantharaj, S., Noda, S., Driess, M. & Menezes, P. W. The pitfalls of using potentiodynamic polarization curves for tafel analysis in electrocatalytic water splitting. ACS Energy Lett. 6, 1607–1611 (2021).
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 11, B1529–B1536 (2010).
Lee, Y., Suntivich, J., May, K. J., Perry, E. E. & Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. L 3, 399–404 (2012).
Ku, H. H. Notes on the use of propagation of error formulas. J. Res. Natl Bur. Stand. 70C, 263–273 (1966).
Payton, M. E., Greenstone, M. H. & Schenker, N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J. Insect Sci. 3, 34 (2003).
Tummers, B. DataThief III. DataThief http://datathief.org (2006).
Rohatgi, A. WebPlotDigitizer version 4.3 https://automeris.io/WebPlotDigitizer/ (2020).
Flower, A., McKenna, J. W. & Upreti, G. Validity and reliability of GraphClick and DataThief III for data extraction. Behav. Modif. 40, 396–413 (2015).
Govindarajan, N., Kastlunger, G., Heenen, H. H. & Chan, K. Improving the intrinsic activity of electrocatalysts for sustainable energy conversion: where are we and where can we go? Chem. Sci. 13, 14–26 (2022).
Winther, K. T. et al. Catalysis-Hub.org, an open electronic structure database for surface reactions. Sci. Data 6, 1–10 (2019).
Álvarez-Moreno, M. et al. Managing the computational chemistry big data problem: the ioChem-BD platform. J. Chem. Inf. Model. 55, 95–103 (2015).
Hummelshøj, J. S., Abild-Pedersen, F., Studt, F., Bligaard, T. & Nørskov, J. K. CatApp: a web application for surface chemistry and heterogeneous catalysis. Angew. Chem. Int. Ed. 51, 272–274 (2012).
Artrith, N. et al. Best practices in machine learning for chemistry. Nat. Chem. 13, 505–508 (2021).
Tran, K. & Ulissi, Z. W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 1, 696–703 (2018).
Zou, X. et al. Machine learning analysis and prediction models of alkaline anion exchange membranes for fuel cells. Energy Environ. Sci. 14, 3965–3975 (2021).
Linpé, W. et al. Revisiting optical reflectance from Au(111) electrode surfaces with combined high-energy surface X-ray diffraction. J. Electrochem. Soc. 168, 096511 (2021).
Resasco, J. et al. Enhancing the connection between computation and experiments in electrocatalysis. Nat. Catal. 5, 374–381 (2022).
Witte, P. T. et al. BASF NanoSelect™ technology: innovative supported Pd- and Pt-based catalysts for selective hydrogenation reactions. Top. Catal. 55, 505–511 (2012).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
McAllister, B. & Hu, P. A density functional theory study of sulfur poisoning. J. Chem. Phys. 122, 084709 (2005).
Akhade, S. A. et al. Poisoning effect of adsorbed CO during CO2 electroreduction on late transition metals. Phys. Chem. Chem. Phys. 16, 20429–20435 (2014).
Yang, G. et al. Interfacial engineering of MoO2–FeP heterojunction for highly efficient hydrogen evolution coupled with biomass electrooxidation. Adv. Mater. 32, 2000455 (2020).
Xie, Y., Zhou, Z., Yang, N. & Zhao, G. An overall reaction integrated with highly selective oxidation of 5-hydroxymethylfurfural and efficient hydrogen evolution. Adv. Funct. Mater. 31, 2102886 (2021).
Wang, Z. et al. Copper–nickel nitride nanosheets as efficient bifunctional catalysts for hydrazine-assisted electrolytic hydrogen production. Adv. Energy Mater. 9, 1900390 (2019).
Chen, Z., Wei, W., Song, L. & Ni, B. Hybrid water electrolysis: a new sustainable avenue for energy-saving hydrogen production. Sustain. Horiz. 1, 100002 (2022). This work introduces fundamentals of hybrid water electrolysis and also provides examples of relevant anodic reactions and catalysts.
Martínez, N. P., Isaacs, M. & Nanda, K. K. Paired electrolysis for simultaneous generation of synthetic fuels and chemicals. New J. Chem. 44, 5617–5637 (2020).
Weinberg, N. L. & Weinberg, H. R. electrochemical oxidation of organic compounds. Chem. Rev. 68, 449–523 (1968).
Xing, L. et al. Platinum electro-dissolution in acidic media upon potential cycling. Electrocatalysis 5, 96–112 (2014).
Furuya, Y. et al. Influence of electrolyte composition and pH on platinum electrochemical and/or chemical dissolution in aqueous acidic media. ACS Catal. 5, 2605–2614 (2015).
Edgington, J., Schweitzer, N., Alayoglu, S. & Seitz, L. C. Constant change: exploring dynamic oxygen evolution reaction catalysis and material transformations in strontium zinc iridate perovskite in acid. J. Am. Chem. Soc. 143, 9961–9971 (2021).
Roy, C. et al. Trends in activity and dissolution on RuO2 versus well-defined extended surfaces. ACS Energy Lett. 3, 2045–2051 (2018).
Cherevko, S. Electrochemical dissolution of noble metals native oxides. J. Electroanal. Chem. 787, 11–13 (2017).
Zhang, R. et al. A dissolution/precipitation equilibrium on the surface of iridium-based perovskites controls their activity as oxygen evolution reaction catalysts in acidic media. Angew. Chem. 131, 4619–4623 (2019).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1012–1014 (2016).
Aßmann, P., Gago, A. S., Gazdzicki, P., Friedrich, K. A. & Wark, M. Toward developing accelerated stress tests for proton exchange membrane electrolyzers. Curr. Opin. Electrochem. 21, 225–233 (2020). This work discusses the importance of accelerated stress tests in the development of electrolysis technology.
Iriawan, H. et al. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Primers 1, 1–26 (2021).
Clark, D. et al. Single-step hydrogen production from NH3, CH4, and biogas in stacked proton ceramic reactors. Science 376, 390–393 (2022).
Lim, D.-K. et al. Solid acid electrochemical cell for the production of hydrogen from ammonia. Joule 4, 2338–2347 (2020).
Shih, A. J. & Haile, S. M. Electrifying membranes to deliver hydrogen. Science 376, 348–349 (2022).
Augustyn, V., Simon, P. & Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014).
Acknowledgements
F.D. and N.L. thank the Spanish Ministry of Science and Innovation (RTI2018-101394-B-I00, Severo Ochoa CEX2019-000925-S). R.M. acknowledges the Dutch Organization for Scientific Research (NWO) for funding under grant number ECCM.TT.ECCM.001. A.H.M.d.S. and R.E.V. acknowledge the Materials Innovation Institute (M2i) and thank Tata Steel Nederland Technology BV and the Dutch Research Council (NWO) (project number ENPPS.IPP.019.002) for financial support. S.P. acknowledges support from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A3A14039678). This project was also supported by the Solar-to-Products programme and the Advanced Research Center for Chemical Building Blocks (ARC CBBC) consortium, both co-financed by the NWO and by Shell Global Solutions B.V., and by the European Commission under contract 722614 (Innovative training network ELCoREL). The authors thank the invaluable peer reviewers who provided constructive comments.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. A.J.S., M.C.O.M., F.D., D.P., M.P., A.H.M.d.S., R.E.V., K.O., S.P., O.v.d.H., G.M., A.G., M.V., G.T.K.K.G., R.M., N.L. and M.T.M.K. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Hydrogen evolution reaction
-
(HER). The reaction at the cathode where hydrogen is produced.
- Oxygen evolution reaction
-
(OER). The reaction at the anode where oxygen is produced.
- Alkaline electrolysis
-
Water splitting under high-pH alkaline conditions. Although water splitting rates are lower under alkaline conditions, cell components exhibit higher resistance against corrosion and catalysts can be prepared from more earth-abundant materials.
- Proton exchange membrane
-
(PEM). A membrane selective towards protons (H+), but not selective towards electrons (insulator) and gases (hydrogen, oxygen).
- Anion exchange membrane
-
A membrane selective towards anions, but not selective towards electrons (insulator), gases (hydrogen, oxygen) and large cations.
- Dimensionally stable anodes
-
Conductive and stable electrodes made of mixed metal oxides (typically of titanium, ruthenium and iridium).
- Reversible hydrogen electrode
-
(RHE). A reference electrode defined as the equilibrium potential of platinum when exposed to 1 atm hydrogen and the pH of the working electrolyte.
- Thiele modulus
-
The ratio of the reaction rate to the diffusion rate.
- Effectiveness factor
-
The ratio of the experimentally measured reaction rate to the kinetic reaction rate in the absence of diffusion limitations.
- Tafel slopes
-
The required increase in potential to increase the reaction rate by ten times.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shih, A.J., Monteiro, M.C.O., Dattila, F. et al. Water electrolysis. Nat Rev Methods Primers 2, 84 (2022). https://doi.org/10.1038/s43586-022-00164-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00164-0
This article is cited by
-
The formation of unsaturated IrOx in SrIrO3 by cobalt-doping for acidic oxygen evolution reaction
Nature Communications (2024)
-
Efficient bubble/precipitate traffic enables stable seawater reduction electrocatalysis at industrial-level current densities
Nature Communications (2024)
-
Data-driven rational design of single-atom materials for hydrogen evolution and sensing
Nano Research (2024)
-
Solutal Marangoni effect determines bubble dynamics during electrocatalytic hydrogen evolution
Nature Chemistry (2023)