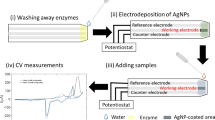
Abstract
Electrochemical stripping analysis (ESA) is a trace electroanalytical technique for the determination of metal cations, inorganic ions, organic compounds and biomolecules. It is based on a pre-concentration step of the target analyte(s), or a compound of the target, on a suitable working electrode. This is followed by a stripping step of the accumulated analyte using an electroanalytical technique. Advantages of ESA include high sensitivity and low limits of detection, multi-analyte capability, low cost of instrumentation and consumables, low power requirements, potential for on-site analysis, speciation capability and scope for indirect biosensing. This Primer covers fundamental aspects of ESA and discusses methods of pre-concentration and stripping, instrumentation, types of working electrodes and sensors, guidelines for method optimization, typical applications, data interpretation and interferences, and method limitations and workarounds. Finally, the current trends and future prospects of ESA are highlighted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Achterberg, P., Barriada, J. L. & Braungardt C. B. in Encyclopedia of Analytical Science 2nd edn (eds Worsfold, P., Townshend, A. & Poole, C.) 203–211 (Elsevier, 2005).
Wang, J. in Comprehensive Analytical Chemistry Vol. 49 (eds Alegret, S. & Merkoçi, A.) 131–141 (Elsevier, 2007).
Munoz, R. A. A., Almeida, E. S. & Angnes, L. in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering 1–11 (Elsevier, 2013).
Adeloju, S. B. in Food Toxicants Analysis (ed. Picó, Y.) 667–696 (Elsevier, 2007).
Alghamdi, A. H. Applications of stripping voltammetric techniques in food analysis. Arab. J. Chem. 3, 1–7 (2010).
Cheraghi, S., Taher, A. M., Bijad, M. & Sadeghifar, H. A review: stripping voltammetric methods as a high sensitive strategy for trace analysis of ions, pharmaceutical and food samples. Curr. Anal. Chem. 13, 5–12 (2017).
Pingarrón, J. M. et al. Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 92, 641–694 (2020).
Vydra, F., Štulík, K. & Juláková, E. Electrochemical Stripping Analysis (E. Horwood, 1976).
Abollino, O., Giacomino, A. & Malandrino, M. in Encyclopedia of Analytical Science 3rd edn Vol. 10 (eds Worsfold, P., Poole, C., Townshend, A. & Miró, M.) 238–257 (Academic, 2019).
Brainina, K. Z. Stripping Voltammetry in Chemical Analysis (Wiley, 1974).
Brainina, K. & Neyman, E. Electroanalytical Stripping Methods (Wiley, 1994).
Wang, J. Stripping Analysis: Principles, Instrumentation and Applications (VCH, 1985).
Economou, A. & Kokkinos, C. in Electrochemical Strategies in Detection Science (ed. Arrigan, D. W. M.) 1–18 (The Royal Society of Chemistry, 2016).
Lovrić, M. in Electroanalytical Methods: Guide to Experiments and Applications (ed. Scholz, F.) 201–218 (Springer, 2010).
Zbinden, C. New method of micro-estimation of the Cu ion. Bull. Soc. Chim. Biol. 13, 35–40 (1931).
Barker, G. C. & Jenkins, I. L. Square-wave polarography. Analyst 77, 685–696 (1952).
Scholz, F. The anfractuous pathways which led to the development of electrochemical stripping techniques. J. Solid. State Electrochem. 15, 1509–1521 (2011).
Daniele, S. in Encyclopedia of Analytical Science 2nd edn (eds Worsfold, P., Townshend, A. & Poole, C.) 197–203 (Elsevier, 2005).
Borrill, A., Reily, N. & Macpherson, J. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: a tutorial review. Analyst 144, 6834–6849 (2019).
Kalvoda, R. & Kopanica, M. Adsorptive stripping voltammetry in trace analysis. Pure Appl. Chem. 61, 97–112 (1989).
Zuhri, A. Z. A. & Voelter, W. Applications of adsorptive stripping voltammetry for the trace analysis of metals, pharmaceuticals and biomolecules. Fresenius J. Anal. Chem. 360, 1–9 (1998).
Hickling, A. A simple potentiostat for general laboratory use. Electrochim. Acta 5, 161–168 (1961).
Colburn, A. W., Levey, K. J., O’Hare, D. & Macpherson, J. V. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 23, 8100–8117 (2021).
Smith, J. & Hinson-Smith, V. Product review: the potentiostat: electrochemistry’s utility player. Anal. Chem. 74, 539 A–541 A (2002).
García-Miranda Ferrari, A., Carrington, P., Rowley-Neale, S. J. & Banks, C. E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 6, 2676–2690 (2020).
Harrington, M. S., Anderson, L. B., Robbins, J. A. & Karweik, D. H. Multiple electrode potentiostat. Rev. Sci. Instrum. 60, 3323–3328 (1989).
Abdullah, S., Serpelloni, M. & Sardini, E. Design of multichannel potentiostat for remote and longtime monitoring of glucose concentration during yeast fermentation. Rev. Sci. Instrum. 91, 054104 (2020).
Rowe, A. A. et al. CheapStat: an open-source, “do-it-yourself” potentiostat for analytical and educational applications. PLoS ONE 6, e23783 (2011).
Economou, A., Bolis, S. D., Efstathiou, C. E. & Volikakis, G. J. A “virtual” electroanalytical instrument for square wave voltammetry. Anal. Chim. Acta 467, 179–188 (2002).
Bezuidenhout, P., Smith, S. & Joubert, T.-H. A low-cost inkjet-printed paper-based potentiostat. Appl. Sci. https://doi.org/10.3390/app8060968 (2018).
Herzog, G. & Arrigan, D. W. M. Determination of trace metals by underpotential deposition–stripping voltammetry at solid electrodes. Trends Analyt. Chem. 24, 208–217 (2005).
Sys, M., Farag, A. & Svancara, I. Extractive stripping voltammetry at carbon paste electrodes for determination of biologically active organic compounds. Monatsh. Fur Chem. 150, 373–386 (2019).
Florence, T. Anodic stripping voltammetry with a glassy carbon electrode mercury-plated in situ. J. Electroanal. Chem. Interfacial Electrochem. 27, 273–281 (1970).
Economou, A. & Fielden, P. R. Mercury film electrodes: developments, trends and potentialities for electroanalysis. Analyst 128, 205–213 (2003).
Economou, A. & Fielden, P. R. Applications, potentialities and limitations of adsorptive stripping analysis on mercury film electrodes. Trends Analyt. Chem. 16, 286–292 (1997).
Mikkelsen, O., Strasunskiene, K., Skogvold, M. S. & Schroder, H. K. Solid alloy electrodes in stripping voltammetry. Curr. Anal. Chem. 4, 202–205 (2008).
Yosypchuk, B. & Barek, J. Analytical applications of solid and paste amalgam electrodes. Crit. Rev. Anal. Chem. 39, 189–203 (2009).
Navrátil, T. et al. in Sensing in Electroanalysis Vol. 6 (eds Metelka, R., Kalcher, K., Švancara, I. & Vytras, K.) 23–53 (University Press Centre, 2011).
Alves, G., Rocha, L. & Soares, H. Multi-element determination of metals and metalloids in waters and wastewaters, at trace concentration level, using electroanalytical stripping methods with environmentally friendly mercury free-electrodes: a review. Talanta 175, 53–68 (2017).
Arino, C., Serrano, N., Diaz-Cruz, J. & Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 990, 11–53 (2017).
Ward Jones, E. S. & Compton, G. R. Stripping analysis using boron-doped diamond electrodes. Curr. Anal. Chem. 4, 170–176 (2008).
Stozhko, N. Y., Malakhova, N. A., Fyodorov, M. V. & Brainina, K. Z. Modified carbon-containing electrodes in stripping voltammetry of metals. J. Solid. State Electrochem. 12, 1185–1204 (2008).
Vytras, K., Svancara, I. & Metelka, R. Carbon paste electrodes in electroanalytical chemistry. J. Serbian Chem. Soc. 74, 1021–1033 (2009).
Zaib, M., Athar, M. M., Saeed, A. & Farooq, U. Electrochemical determination of inorganic mercury and arsenic — a review. Biosens. Bioelectron. 74, 895–908 (2015).
Alves, G. M. S., Magalhães, J. M. C. S., Salaün, P., van den Berg, C. M. G. & Soares, H. M. V. M. Simultaneous electrochemical determination of arsenic, copper, lead and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Anal. Chim. Acta 703, 1–7 (2011).
García-Miranda Ferrari, A., Rowley-Neale, S. J. & Banks, C. E. Screen-printed electrodes: transitioning the laboratory in-to-the field. Talanta Open 3, 100032 (2021).
Economou, A. Screen-printed electrodes modified with “Green” metals for electrochemical stripping analysis of toxic elements. Sensors https://doi.org/10.3390/s18041032 (2018).
Niu, X. et al. Review: electrochemical stripping analysis of trace heavy metals using screen-printed electrodes. Anal. Lett. 46, 2479–2502 (2013).
Honeychurch, K. Screen-printed electrochemical sensors and biosensors for monitoring metal pollutants. Insciences J. 2, 1–51 (2012).
Pitsou, M. et al. “Green” three-electrode sensors fabricated by injection-moulding for on-site stripping voltammetric determination of trace In(III) and Tl(I). Chemosensors https://doi.org/10.3390/chemosensors9110310 (2021).
Gao, W. et al. Wearable microsensor array for multiplexed heavy metal monitoring of body fluids. ACS Sens. 1, 866–874 (2016).
Zhang, W., Zhang, H., Williams, S. E. & Zhou, A. Microfabricated three-electrode on-chip PDMS device with a vibration motor for stripping voltammetric detection of heavy metal ions. Talanta 132, 321–326 (2015).
Baracu, A. M. & Dinu Gugoasa, L. A. Review — recent advances in microfabrication, design and applications of amperometric sensors and biosensors. J. Electrochem. Soc. 168, 037503 (2021).
Roditi, E., Tsetsoni, M., Kokkinos, C. & Economou, A. Integrated on-chip sensor with sputtered Ag–Au–Au electrodes for the voltammetric determination of trace Hg(II). Sens. Actuators B Chem. 286, 125–130 (2019).
Lee, K. Y., Ambrosi, A. & Pumera, M. 3D-printed metal electrodes for heavy metals detection by anodic stripping voltammetry. Electroanalysis 29, 2444–2453 (2017).
Walters, J. G., Ahmed, S., Terrero Rodríguez, I. M. & O’Neil, G. D. Trace analysis of heavy metals (Cd, Pb, Hg) using native and modified 3D printed graphene/poly(lactic acid) composite electrodes. Electroanalysis 32, 859–866 (2020).
Economou, A. Recent developments in on-line electrochemical stripping analysis — an overview of the last 12 years. Anal. Chim. Acta 683, 38–51 (2010).
Baltima, A., Panagopoulou, H., Economou, A. & Kokkinos, C. 3D-printed fluidic electrochemical microcell for sequential injection/stripping analysis of heavy metals. Anal. Chim. Acta 1159, 338426 (2021).
Zou, Z. et al. Environmentally friendly disposable sensors with microfabricated on-chip planar bismuth electrode for in situ heavy metal ions measurement. Sens. Actuators B Chem. 134, 18–24 (2008).
Gharib Naseri, N., Baldock, S. J., Economou, A., Goddard, N. J. & Fielden, P. R. Disposable electrochemical flow cells for catalytic adsorptive stripping voltammetry (CAdSV) at a bismuth film electrode (BiFE). Anal. Bioanal. Chem. 391, 1283–1292 (2008).
Shen, L.-L., Zhang, G.-R., Li, W., Biesalski, M. & Etzold, B. J. M. Modifier-free microfluidic electrochemical sensor for heavy-metal detection. ACS Omega 2, 4593–4603 (2017).
Kokkinos, C. T., Giokas, D. L., Economou, A. S., Petrou, P. S. & Kakabakos, S. E. Paper-based microfluidic device with integrated sputtered electrodes for stripping voltammetric determination of DNA via quantum dot labeling. Anal. Chem. 90, 1092–1097 (2018). This work combines three emerging trends in ESA: microengineering fabrication, paper support and nanomaterial-based ESA bioassay.
Herzog, G. & Beni, V. Stripping voltammetry at micro-interface arrays: a review. Anal. Chim. Acta 769, 10–21 (2013).
Daniele, S., Baldo, A. M. & Bragato, C. Recent developments in stripping analysis on microelectrodes. Curr. Anal. Chem. 4, 215–228 (2008).
Beni, V. & Arrigan, W. M. D. Microelectrode arrays and microfabricated devices in electrochemical stripping analysis. Curr. Anal. Chem. 4, 229–241 (2008).
Gouveia-Caridade, C. & Brett, M. A. C. Strategies, development and applications of polymer-modified electrodes for stripping analysis. Curr. Anal. Chem. 4, 206–214 (2008).
Ward Jones, E. S. & Compton, G. R. Fabrication and applications of nanoparticle-modified electrodes in stripping analysis. Curr. Anal. Chem. 4, 177–182 (2008).
Metters, J. P. & Banks, C. E. in Nanosensors for Chemical and Biological Applications (ed. Honeychurch, K. C.) 54–79 (Woodhead, 2014).
Lu, Y. et al. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 178, 324–338 (2018).
Chapman, C. S. & van den Berg, C. M. G. Anodic stripping voltammetry using a vibrating electrode. Electroanalysis 19, 1347–1355 (2007).
Grundler, P. Stripping analysis enhanced by ultrasound, electrode heating and magnetic fields. Curr. Anal. Chem. 4, 263–270 (2008).
Rodrigues, J. A. et al. Increased sensitivity of anodic stripping voltammetry at the hanging mercury drop electrode by ultracathodic deposition. Anal. Chim. Acta 701, 152–156 (2011).
Salaün, P., Gibbon-Walsh, K. & van den Berg, C. M. G. Beyond the hydrogen wave: new frontier in the detection of trace elements by stripping voltammetry. Anal. Chem. 83, 3848–3856 (2011).
Anson, F. C. Patterns of ionic and molecular adsorption at electrodes. Acc. Chem. Res. 8, 400–407 (1975).
Scholz, F. Voltammetric techniques of analysis: the essentials. ChemTexts 1, 17 (2015).
Laborda, E., González, J. & Molina, Á. Recent advances on the theory of pulse techniques: a mini review. Electrochem. Commun. 43, 25–30 (2014).
Carter, M. T. & Osteryoung, R. A. in Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation (eds Meyers, R. A., White H. S. & Crooks, R. M.) (Wiley, 2006).
Honeychurch, M. J., Díaz-Cruz, J. M., Serrano, N., Ariño, C. & Esteban, M. in Encyclopedia of Analytical Science 3rd edn (eds Worsfold, P., Poole, C., Townshend, A. & Miró, M.) 230–237 (Academic, 2019).
Serrano, N., Diaz-Cruz, J., Arino, C. & Esteban, M. Stripping chronopotentiometry in environmental analysis. Electroanalysis 19, 2039–2049 (2007).
Soares, H. M. V. M. & Vasconcelos, M. T. S. D. Potentiometric stripping analysis vs. differential pulse anodic stripping voltammetry for copper(II) analysis at relatively positive deposition potential. Anal. Chim. Acta 303, 255–263 (1995).
Coetzee, J. F. & Ecoff, M. J. Potentiometric stripping analysis at microelectrodes in various solvents and some comparisons with voltammetric stripping analysis. Anal. Chem. 63, 957–963 (1991).
Town, R. M. Potentiometric stripping analysis and anodic stripping voltammetry for measurement of copper(II) and lead(II) complexation by fulvic acid: a comparative study. Electroanalysis 9, 407–415 (1997).
Omanović, D., Garnier, C., Gibbon–Walsh, K. & Pižeta, I. Electroanalysis in environmental monitoring: tracking trace metals — a mini review. Electrochem. Commun. 61, 78–83 (2015).
Buffle, J. & Chalmers, R. A. Complexation reactions in aquatic systems: an analytical approach (Ellis Horwood, 1988).
Han, H. & Pan, D. Voltammetric methods for speciation analysis of trace metals in natural waters. Trends Environ. Anal. Chem. 29, e00119 (2021).
Bott, A. W. Voltammetric determination of trace concentrations of metals in the environment. Curr. Sep. 14, 24–30 (1995).
Namieśnik, J. & Rabajczyk, A. The speciation and physico-chemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 22, 1–24 (2010).
Wasiewska, L. A. et al. Reagent free electrochemical-based detection of silver ions at interdigitated microelectrodes using in-situ pH control. Sens. Actuators B Chem. 333, 129531 (2021).
Rotureau, E. et al. Electroanalytical metal sensor with built-in oxygen filter. Anal. Chim. Acta 1167, 338544 (2021).
Yu, X.-L. & He, Y. Application of Box–Behnken designs in parameters optimization of differential pulse anodic stripping voltammetry for lead(II) determination in two electrolytes. Sci. Rep. 7, 2789 (2017).
Tarley, C. R. T. et al. Chemometric tools in electroanalytical chemistry: methods for optimization based on factorial design and response surface methodology. Microchem. J. 92, 58–67 (2009).
Furlanetto, S., Pinzauti, S., Gratteri, P., La Porta, E. & Calzeroni, G. Experimental design strategies in the optimization and robustness testing of adsorptive stripping voltammetric conditions for kynurenic acid determination. J. Pharm. Biomed. Anal. 15, 1585–1594 (1997).
Pinto, L. & Lemos, S. G. Multivariate optimization of the voltammetric determination of Cd, Cu, Pb and Zn at bismuth film. Application to analysis of biodiesel. Microchem. J. 110, 417–424 (2013).
Buffle, J. & Tercier-Waeber, M. L. Voltammetric environmental trace-metal analysis and speciation: from laboratory to in situ measurements. Trends Anal. Chem. 24, 172–191 (2005).
Jagner, D. & Graneli, A. Potentiometric stripping analysis. Anal. Chim. Acta 83, 19–26 (1976). This work conceptualizes PSA.
Serrano, N., Díaz-Cruz, J. M., Ariño, C. & Esteban, M. Comparison of constant-current stripping chronopotentiometry and anodic stripping voltammetry in metal speciation studies using mercury drop and film electrodes. J. Electroanal. Chem. 560, 105–116 (2003).
Stromberg, A. G. & Romanenko, S. V. Determination of the true form of overlapping peaks, deformed by the base line in the case of stripping voltammetry. Fresenius J. Anal. Chem. 361, 276–279 (1998).
Romanenko, S. V., Romanenko, E. S. & Kolpakova, N. A. Use of a spline function of a fractional degree for the description of the base line in the determination of platinum by stripping voltammetry. J. Anal. Chem. 56, 51–55 (2001).
Górski, Ł., Jakubowska, M., Baś, B. & Kubiak, W. W. Application of genetic algorithm for baseline optimization in standard addition voltammetry. J. Electroanal. Chem. 684, 38–46 (2012).
Cobelo-García, A., Santos-Echeandía, J., López-Sánchez, D. E., Almécija, C. & Omanović, D. Improving the voltammetric quantification of ill-defined peaks using second derivative signal transformation: example of the determination of platinum in water and sediments. Anal. Chem. 86, 2308–2313 (2014).
Economou, A., Fielden, P. R. & Packham, A. J. Data smoothing in stripping voltammetry by simplex function fitting. Anal. Lett. 30, 2595–2610 (1997).
Economou, A. & Fielden, P. R. Digital filtering in stripping analysis. Anal. Chim. Acta 305, 165–175 (1995).
Díaz-Cruz, J. M., Esteban, M. & Ariño, C. Chemometrics in Electroanalysis (Springer, 2019).
Prikler, S. & Einax, J. W. Wavelet transform as a new approach to the enhancement of signal-to-noise ratio in anodic stripping voltammetry. Anal. Bioanal. Chem. 395, 1707 (2009).
Economou, A., Fielden, P. R., Gaydecki, P. A. & Packham, A. J. Data enhancement in adsorptive stripping voltammetry by the application of digital signal processing techniques. Analyst 119, 847–853 (1994).
Romanenko, S. V., Larin, S. L. & Stasyuk, N. V. Use of differentiation and smoothing in linear-sweep and staircase stripping voltammetry of some metals. J. Anal. Chem. 55, 1063–1068 (2000).
Jakubowska, M. Signal processing in electrochemistry. Electroanalysis 23, 553–572 (2011). This work presents a comprehensive review of data evaluation methods in electrochemistry.
Akrami, A., Niazi, A. & Bagheban-Shahri, F. Determination of trace amounts of gold in environmental samples by adsorptive stripping voltammetry of its complex with rhodamine using Osc-Pls. J. Chem. Health Risks https://doi.org/10.22034/jchr.2018.544004 (2012).
Melucci, D. & Locatelli, C. Multivariate calibration in differential pulse stripping voltammetry using a home-made carbon-nanotubes paste electrode. J. Electroanal. Chem. 675, 25–31 (2012).
Galeano-Díaz, T., Guiberteau-Cabanillas, A., Espinosa-Mansilla, A. & López-Soto, M. D. Adsorptive stripping square wave voltammetry (Ad-SSWV) accomplished with second-order multivariate calibration: determination of fenitrothion and its metabolites in river water samples. Anal. Chim. Acta 618, 131–139 (2008).
Galeano-Díaz, T., Acedo-Valenzuela, M.-I., Mora-Diez, N. & Silva-Rodríguez, A. Simultaneous differential pulse adsorptive stripping determination of imipramine and its metabolite desipramine by the PLS-1 multivariate method. Electroanalysis 23, 449–455 (2011).
Ni, Y., Qiu, P. & Kokot, S. Simultaneous determination of three organophosphorus pesticides by differential pulse stripping voltammetry and chemometrics. Anal. Chim. Acta 516, 7–17 (2004).
Esteban, M., Ariño, C. & Díaz-Cruz, J. M. Chemometrics in electroanalytical chemistry. Crit. Rev. Anal. Chem. 36, 295–313 (2006).
Zhang, J.-q. et al. In situ fast analysis of cadmium in rice by diluted acid extraction-anodic stripping voltammetry. RSC Adv. 9, 19965–19972 (2019).
Whang, C. W., Page, J. A., VanLoon, G. & Griffin, M. P. Modified standard additions calibration for anodic stripping voltammetry. Anal. Chem. 56, 539–542 (1984).
Gerlach, R. W. & Kowalski, B. R. The generalized standard addition method: intermetallic interferences in anodic stripping voltammetry. Anal. Chim. Acta 134, 119–127 (1982).
Fernando, A. R. & Kratochvil, B. Internal standards in differential pulse anodic stripping voltammetry. Can. J. Chem. 69, 755–758 (1991).
Brown, R. J. C., Roberts, M. R. & Brett, D. J. L. Stripping voltammetry using sequential standard addition calibration with the analytes themselves acting as internal standards. Anal. Chim. Acta 635, 1–5 (2009).
Wang, J., Anik Kirgöz, Ü. & Lu, J. Stripping voltammetry with the electrode material acting as a ‘built-in’ internal standard. Electrochem. Commun. 3, 703–706 (2001).
Ellison, S. L. R. & Thompson, M. Standard additions: myth and reality. Analyst 133, 992–997 (2008).
Monticelli, D., Ciceri, E. & Dossi, C. Optimization and validation of an automated voltammetric stripping technique for ultratrace metal analysis. Anal. Chim. Acta 594, 192–198 (2007).
Ozkan, S. A., Kauffmann, J.-M. & Zuman, P. Electroanalysis in Biomedical and Pharmaceutical Sciences: Voltammetry, Amperometry, Biosensors, Applications (eds Ozkan, S. A., Kauffmann, J.-M. & Zuman, P.) 235–266 (Springer, 2015).
Gustavo González, A. & Ángeles Herrador, M. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. Trends Anal. Chem. 26, 227–238 (2007).
Wang, J. Analytical Electrochemistry 2nd edn (Wiley-VCH, 2000).
Brainina, K., Malakhova, N. & Stozhko, N. Stripping voltammetry in environmental and food analysis. Fresenius J. Anal. Chem. 368, 307–325 (2000).
Thomas, F. G. & Henze, G. Introduction to Voltammetric Analysis: Theory and Practice (CSIRO, 2001).
Bulska, E. & Ruszczyńska, A. Analytical techniques for trace element determination. Phys. Sci. Rev. https://doi.org/10.1515/psr-2017-8002 (2017).
Gustavsson, I. & Hansson, L. Intercomparison studies of stripping voltammetry and atomic absorption spectrometry of Zn, Cd, Pb, Cu, Ni and Co in Baltic sea water. Int. J. Environ. Anal. Chem. 17, 57–72 (1984).
Rodriguez Villarreal, K., Alva, A., Santos, Z. V. & Roman-Gonzalez, A. Comparative study of methods that detect levels of lead and its consequent toxicity in the blood. Int. J. Adv. Comput. Sci. Appl. https://doi.org/10.14569/IJACSA.2019.0100664 (2019).
World Health Organization. Brief Guide to Analytical Methods for Measuring Lead in Blood 2nd edn, 14 (World Health Organization, 2020).
Niedzielski, P. & Siepak, M. Analytical methods for determining arsenic, antimony and selenium in environmental samples. Pol. J. Environ. Stud. 12, 653–667 (2003).
Cartier, C., Bannier, A., Pirog, M., Nour, S. & Prévost, M. A rapid method for lead service line detection. J. AWWA 104, E596–E607 (2012).
Taylor, L. et al. Evaluation of a portable blood lead analyzer with occupationally exposed populations. Am. J. Ind. Med. 40, 354–362 (2001).
Nakata, H. et al. Assessment of LeadCare® II analysis for testing of a wide range of blood lead levels in comparison with ICP-MS analysis. Chemosphere https://doi.org/10.1016/j.chemosphere.2021.129832 (2021).
Ortiz, M. et al. Lead in candy consumed and blood lead levels of children living in Mexico City. Environ. Res. 147, 497–502 (2016).
Boesen, A. et al. Assessment of the LeadCare® Plus for use on Scandinavian brown bears (Ursus arctos). Front. Vet. Sci. https://doi.org/10.3389/fvets.2019.00285 (2019).
Brown, C., Luebbert, J., Mulcahy, D., Schamber, J. & Rosenberg, D. Blood lead levels of wild Steller’s eiders (Polysticta stelleri) and black scoters (Melanitta nigra) in Alaska using a portable blood lead analyzer. J. Zoo. Wildl. Med. 37, 361–365 (2006).
Serrano, N., Alberich, A., Diaz-Cruz, J., Arino, C. & Esteban, M. Coating methods, modifiers and applications of bismuth screen-printed electrodes. Trends Anal. Chem. 46, 15–29 (2013).
March, G., Nguyen, T. & Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 5, 241–275 (2015).
Holmes, J., Pathirathna, P. & Hashemi, P. Novel frontiers in voltammetric trace metal analysis: towards real time, on-site, in situ measurements. Trends Anal. Chem. 111, 206–219 (2019).
van den Berg, C. Potentials and potentialities of cathodic stripping voltammetry of trace-elements in natural-waters. Anal. Chim. Acta 250, 265–276 (1991).
van den Berg, C. M. G. in Methods of Seawater Analysis (eds Grasshoff, K., Kremling, K. & Ehrhardt, M.) 302–318 (Wiley, 1999).
Pihlar, B., Valenta, P. & Nürnberg, H. New high-performance analytical procedure for the voltammetric determination of nickel in routine analysis of waters, biological materials and food. Fresenius Z. Anal. Chem. 307, 337–346 (1981).
Tercier, M. & Buffle, J. Insitu voltammetric measurements in natural-waters — future-prospects and challenges. Electroanalysis 5, 187–200 (1993).
Paneli, M. & Voulgaropoulos, A. Applications of adsorptive stripping voltammetry in the determination of trace and ultratrace metals. Electroanalysis 5, 355–373 (1993).
Achterberg, E. & Braungardt, C. Stripping voltammetry for the determination of trace metal speciation and in-situ measurements of trace metal distributions in marine waters. Anal. Chim. Acta 400, 381–397 (1999).
Czae, M. & Wang, J. Pushing the detectability of voltammetry: how low can we go? Talanta 50, 921–928 (1999).
Bobrowski, A. & Zarebski, J. Catalytic systems in adsorptive stripping voltammetry. Electroanalysis 12, 1177–1186 (2000). This work presents a comprehensive review of catalytic systems in ESA.
Bobrowski, A. & Zarebski, J. Catalytic adsorptive stripping voltammetry at film electrodes. Curr. Anal. Chem. 4, 191–201 (2008).
Bobrowski, A., Krolicka, A. & Zarebski, J. Characteristics of voltammetric determination and speciation of chromium — a review. Electroanalysis 21, 1449–1458 (2009).
Bobrowski, A. & Zarebski, J. Review of the catalytic voltammetric determination of titanium traces. Acta Chim. Slov. 59, 233–241 (2012).
Wang, J. et al. Stripping analysis into the 21st century: faster, smaller, cheaper, simpler and better. Anal. Chim. Acta 385, 429–435 (1999).
Tercier-Waeber, M. et al. A novel probe and microsensors for in situ, continuous, automatic profiling of trace elements in natural waters. Oceans’98 — Conf. Proc. 1–3, 956–959 (1998).
Howell, K. et al. Voltammetric in situ measurements of trace metals in coastal waters. Trends Anal. Chem. 22, 828–835 (2003).
Tercier, M., Buffle, J. & Graziottin, F. Novel voltammetric in-situ profiling system for continuous real-time monitoring of trace elements in natural waters. Electroanalysis 10, 355–363 (1998).
Wang, J. et al. Flow probe for in situ electrochemical monitoring of trace chromium. Analyst 124, 349–352 (1999).
Wang, J. et al. Remote electrochemical sensor for monitoring trace mercury. Electroanalysis 10, 399–402 (1998).
Wang, J. et al. Renewable-reagent electrochemical sensor for monitoring trace metal contaminants. Anal. Chem. 69, 2640–2645 (1997).
Romanus, A., Muller, H. & Kirsch, D. Application of adsorptive stripping voltammetry (AdSV) for the analysis of trace-metals in brine.1. Batch voltammetric measurements. Fresenius J. Anal. Chem. 340, 363–370 (1991).
Romanus, A., Muller, H. & Kirsch, D. Application of adsorptive stripping voltammetry (AdSV) for the analysis of trace-metals in brine. 2. Development and evaluation of a flow-injection system. Fresenius J. Anal. Chem. 340, 371–376 (1991).
O’Mahony, A. M. & Wang, J. Electrochemical detection of gunshot residue for forensic analysis: a review. Electroanalysis 25, 1341–1358 (2013).
Santos, A., Takeuchi, R., Fenga, P. & Ramos, S. in Applications and Experiences of Quality Control (ed. Ivanov, O.) 451–495 (InTech, 2011).
Wygant, B. R. & Lambert, T. N. Thin film electrodes for anodic stripping voltammetry: a mini-review. Front. Chem. https://doi.org/10.3389/fchem.2021.809535 (2022).
Sanga, N. A., Jahed, N., Leve, Z., Iwuoha, E. I. & Pokpas, K. Simultaneous adsorptive stripping voltammetric analysis of heavy metals at graphenated cupferron pencil rods. J. Electrochem. Soc. 169, 017502 (2022).
Mettakoonpitak, J., Volckens, J. & Henry, C. S. Janus electrochemical paper-based analytical devices for metals detection in aerosol samples. Anal. Chem. 92, 1439–1446 (2020). This work presents a modern multiplexed approach for the determination of several trace metals by ESA on a paper-based device.
Florence, T. Electrochemical approaches to trace-element speciation in waters — a review. Analyst 111, 489–505 (1986).
Florence, T. Trace-element speciation by anodic-stripping voltammetry. Analyst 117, 551–553 (1992).
Monticelli, D. & Caprara, S. Voltammetric tools for trace element speciation in fresh waters: methodologies, outcomes and future perspectives. Environ. Chem. 12, 683–705 (2015).
Companys, E., Galceran, J., Pinheiro, J., Puy, J. & Salaun, P. A review on electrochemical methods for trace metal speciation in environmental media. Curr. Opin. Electrochem. 3, 144–162 (2017). This work presents a comprehensive review of metal speciation methods using ESA.
Town, R. & van Leeuwen, H. Stripping chronopotentiometry at scanned deposition potential (SSCP): an effective methodology for dynamic speciation analysis of nanoparticulate metal complexes. J. Electroanal. Chem. https://doi.org/10.1016/j.jelechem.2019.113530 (2019).
Korolczuk, M. & Rutyna, I. New methodology for anodic stripping voltammetric determination of methylmercury. Electrochem. Commun. 10, 1024–1026 (2008).
Van den Berg, C. Organic and inorganic speciation of copper in the Irish Sea. Mar. Chem. 14, 201–212 (1984).
Bas, B. Refreshable mercury film silver based electrode for determination of chromium(VI) using catalytic adsorptive stripping voltammetry. Anal. Chim. Acta 570, 195–201 (2006).
Bobrowski, A., Kapturski, P. & Zarebski, J. Catalytic adsorptive stripping chronopotentiometric determination of hexavalent chromium at a silver amalgam film electrode of prolonged application. Electroanalysis 23, 2265–2269 (2011).
Espada-Bellido, E., Bi, Z. & van den Berg, C. Determination of chromium in estuarine waters by catalytic cathodic stripping voltammetry using a vibrating silver amalgam microwire electrode. Talanta 105, 287–291 (2013).
Lin, L., Lawrence, N., Thongngamdee, S., Wang, J. & Lin, Y. Catalytic adsorptive stripping determination of trace chromium(VI) at the bismuth film electrode. Talanta 65, 144–148 (2005).
Yong, L. et al. Quantitative analysis of trace chromium in blood samples. Combination of the advanced oxidation process with catalytic adsorptive stripping voltammetry. Anal. Chem. 78, 7582–7587 (2006).
Jorge, E., Rocha, M., Fonseca, I. & Neto, M. Studies on the stripping voltammetric determination and speciation of chromium at a rotating-disc bismuth film electrode. Talanta 81, 556–564 (2010).
Hue, N. et al. Determination of chromium in natural water by adsorptive stripping voltammetry using in situ bismuth film electrode. J. Environ. Public Health https://doi.org/10.1155/2020/1347836 (2020).
Ouyang, R. et al. Improved Bi film wrapped single walled carbon nanotubes for ultrasensitive electrochemical detection of trace Cr(VI). Electrochim. Acta 113, 686–693 (2013).
Bond, A. 200 years of practical electroanalytical chemistry: past, present and future directions illustrated by reference to the on-line, on-stream and off-line determination of trace metals in zinc plant electrolyte by voltammetric and potentiometric techniques. Anal. Chim. Acta 400, 333–379 (1999).
Bobrowski, A. & Bond, A. Catalytic adsorptive stripping voltammetric determination of cobalt as an α-benzil dioxime complex in the presence of an extremely large excess of zinc. Electroanalysis 3, 157–162 (1991).
Mrzljak, R. et al. Online monitoring of cobalt in zinc plant electrolyte by differential-pulse adsorptive stripping voltammetry. Anal. Chim. Acta 281, 281–290 (1993).
Zarebski, J. Studies on some catalytic-systems by means of polarographic and voltammetric methods 2. Chem. Anal. 32, 223–232 (1987).
Golimowski, J., Valenta, P. & Nurnberg, H. Trace determination of chromium in various water types by adsorption differential pulse voltammetry. Fresenius Z. Anal. Chem. 322, 315–322 (1985).
Boussemart, M., van den Berg, C. & Ghaddaf, M. The determination of the chromium speciation in sea-water using catalytic cathodic stripping voltammetry. Anal. Chim. Acta 262, 103–115 (1992).
Sander, S. & Koschinsky, A. Onboard-ship redox speciation of chromium in diffuse hydrothermal fluids from the North Fiji Basin. Mar. Chem. 71, 83–102 (2000).
Li, Y. & Xue, H. Determination of Cr(III) and Cr(VI) species in natural waters by catalytic cathodic stripping voltammetry. Anal. Chim. Acta 448, 121–134 (2001).
Sander, S., Navratil, T. & Novotny, L. Study of the complexation, adsorption and electrode reaction mechanisms of chromium(VI) and (III) with DTPA under adsorptive stripping voltammetric conditions. Electroanalysis 15, 1513–1521 (2003).
Bobrowski, A. et al. Chromium speciation study in polluted waters using catalytic adsorptive stripping voltammetry and tangential flow filtration. Talanta 63, 1003–1012 (2004).
Grabarczyk, M., Korolczuk, M. & Kaczmarek, L. Simple and highly selective catalytic adsorptive voltammetric method for Cr(VI) determination. Electroanalysis 18, 2381–2384 (2006).
Smyth, W. & Smyth, M. Electrochemical analysis of organic pollutants. Pure Appl. Chem. 59, 245–256 (1987).
Smyth, W. Voltammetric determination of molecules of biological, environmental, and pharmaceutical importance. CRC Crit. Rev. Anal. Chem. 18, 155–208 (1987).
Barek, J., Peckova, K. & Vyskocil, V. Adsorptive stripping voltammetry of environmental carcinogens. Curr. Anal. Chem. 4, 242–249 (2008).
Vesela, H. & Šucman, E. Determination of acrylamide in food using adsorption stripping voltammetry. Czech. J. Food Sci. 31, 401–406 (2013).
Nissim, R. & Compton, R. G. Absorptive stripping voltammetry for cannabis detection. Chem. Cent. J. 9, 41 (2015).
Prabu, H., Talawar, M., Mukundan, T. & Asthana, S. Studies on the utilization of stripping voltammetry technique in the detection of high-energy materials. Combust. Explos. Shock Waves 47, 87–95 (2011).
Palecek, E., Heyrovsky, M. & Dorcak, V. J. Heyrovsky’s oscillographic polarography. roots of present chronopotentiometric analysis of biomacromolecules. Electroanalysis 30, 1259–1270 (2018).
Fojta, M., Jelen, F., Havran, L. & Palecek, E. Electrochemical stripping techniques in analysis of nucleic acids and their constituents. Curr. Anal. Chem. 4, 250–262 (2008). This work is a direct analysis of nucleic acids using ESA.
Ozkan, S., Uslu, B. & Aboul-Enein, H. Analysis of pharmaceuticals and biological fluids using modern electroanalytical techniques. Crit. Rev. Anal. Chem. 33, 155–181 (2003).
Kul, D. Voltammetric analysis of atypical antipsychotic drugs with solid electrodes. Curr. Anal. Chem. 15, 240–248 (2019).
Gupta, V., Jain, R., Radhapyari, K., Jadon, N. & Agarwal, S. Voltammetric techniques for the assay of pharmaceuticals — a review. Anal. Biochem. 408, 179–196 (2011).
Goncalves, D., Silva, C. & De Souza, D. Pesticides determination in foods and natural waters using solid amalgam-based electrodes: challenges and trends. Talanta https://doi.org/10.1016/j.talanta.2020.120756 (2020).
Lezi, N., Economopoulos, S., Prodromidis, M., Economou, A. & Tagmatarchis, N. Fabrication of a “green” and low-cost screen-printed graphene sensor and its application to the determination of caffeine by adsorptive stripping voltammetry. Int. J. Electrochem. Sci. 12, 6054–6067 (2017).
Gratteri, P. et al. Adsorptive stripping voltammetry for thiomersal assay. J. Pharm. Biomed. Anal. 12, 273–276 (1994).
Piech, R., Wymazala, J., Smajdor, J. & Paczosa-Bator, B. Thiomersal determination on a renewable mercury film silver-based electrode using adsorptive striping voltammetry. Anal. Methods 8, 1187–1193 (2016).
Penagos-Llanos, J., Calderon, J., Nagles, E. & Hurtado, J. Voltammetric determination of thiomersal with a new modified electrode based on a carbon paste electrode decorated with La2O3. J. Electroanal. Chem. 833, 536–542 (2019).
Gonzalez, C., Garcia-Beltran, O. & Nagles, E. A new and simple electroanalytical method to detect thiomersal in vaccines on a screen-printed electrode modified with chitosan. Anal. Methods 10, 1196–1202 (2018).
Kokkinos, C. & Economou, A. Emerging trends in biosensing using stripping voltammetric detection of metal-containing nanolabels — a review. Anal. Chim. Acta 961, 12–32 (2017).
Valera, E., Hernández-Albors, A. & Marco, M. P. Electrochemical coding strategies using metallic nanoprobes for biosensing applications. Trends Anal. Chem. 79, 9–22 (2016).
Qi, W., Wu, D., Xu, G., Nsabimana, J. & Nsabimana, A. Aptasensors based on stripping voltammetry. Chemosensors 4, 12 (2016).
Feng, J., Chu, C. & Ma, Z. Electrochemical signal substance for multiplexed immunosensing interface construction: a mini review. Molecules https://doi.org/10.3390/molecules27010267 (2022).
Ellison, S. L. R. & Williams, A. (eds) Eurachem/CITAC guide: metrological traceability in analytical measurement: a guide to achieving comparable results in chemical measurement, 2nd edn. Eurachem https://www.eurachem.org/images/stories/Guides/pdf/ECTRC_2019_EN_P1.pdf (2019).
Miller, J. C. & Miller, J. N. Statistics and Chemometrics for Analytical Chemistry 6th edn (Pearson, 2010).
Pérez-Ràfols, C., Serrano, N., Diaz-Cruz, J. M., Arino, C. & Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Anal. Chim. Acta 916, 17–23 (2016).
Han, X., Meng, Z., Zhang, H. & Zheng, J. Fullerene-based anodic stripping voltammetry for simultaneous determination of Hg(II), Cu(II), Pb(II) and Cd(II) in foodstuff. Microchim. Acta 185, 274 (2018).
Wang, J. Analytical Electrochemistry 3rd edn (Wiley, 2006).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods. Fundamentals and Applications 2nd edn (Wiley, 2001).
Hanssen, B. L., Siraj, S. & Wong, D. K. Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 35, 1–28 (2016).
Hackel, L., Rotureau, E., Morrin, A. & Pinheiro, J. P. Developing on-site trace level speciation of lead, cadmium and zinc by stripping chronopotentiometry (SCP): fast screening and quantification of total metal concentrations. Molecules https://doi.org/10.3390/molecules26185502 (2021).
Mart, L., Nürnberg, H. W. & Valenta, P. Prevention of contamination and other accuracy risks in voltammetric trace metal analysis of natural waters. Fresenius Z. Anal. Chem. 300, 350–362 (1980).
Wang, J., Lu, J., Hocevar, S. B., Farias, P. A. M. & Ogorevc, B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 72, 3218–3222 (2000). This work introduces the first ‘green’ electrode material (bismuth) in ESA.
Stulík, K., Amatore, C., Holub, K., Marecek, V. & Kutner, W. Microelectrodes. Definitions, characterization, and applications (technical report). Pure Appl. Chem. 72, 1483–1492 (2000).
Mohamad Nor, N., Ramli, N. H., Poobalan, H., Qi Tan, K. & Abdul Razak, K. Recent advancement in disposable electrode modified with nanomaterials for electrochemical heavy metal sensors. Crit. Rev. Anal. Chem. https://doi.org/10.1080/10408347.2021.1950521 (2021).
Bott, A. W. Practical problems in voltammetry: 4. Preparation of working electrodes. Curr. Sep. 16, 79–84 (1997).
Bobrowski, A., Królicka, A. & Bobrowski, R. Renewable silver amalgam film electrodes in electrochemical stripping analysis — a review. J. Solid. State Electrochem. 20, 3217–3228 (2016).
Baś, B., Węgiel, K. & Jedlińska, K. The renewable bismuth bulk annular band working electrode: fabrication and application in the adsorptive stripping voltammetric determination of nickel(II) and cobalt(II). Anal. Chim. Acta 881, 44–53 (2015).
Porada, R., Jedlińska, K., Lipińska, J. & Baś, B. Review — voltammetric sensors with laterally placed working electrodes: a review. J. Electrochem. Soc. 167, 037536 (2020).
Copeland, T. R., Osteryoung, R. A. & Skogerboe, R. K. Elimination of copper–zinc intermetallic interferences in anodic stripping voltammetry. Anal. Chem. 46, 2093–2097 (1974).
Oviedo, O. A., Reinaudi, L., García, S. G. & Leiva, E. P. M. Underpotential Deposition. From Fundamentals and Theory to Applications at the Nanoscale. Series: Monographs in Electrochemistry. J. Solid State Electrochem. 20, 2383–2385 (2016).
Kadara, R. O. & Tothill, I. E. Resolving the copper interference effect on the stripping chronopotentiometric response of lead(II) obtained at bismuth film screen-printed electrode. Talanta 66, 1089–1093 (2005).
Scollary, G. R. et al. Elimination of zinc/copper intermetallic interference in the determination of zinc in wastewaters and seawater by potentiometric stripping analysis. Electroanalysis 5, 685–689 (1993).
Neiman, E. Y., Petrova, L. G., Ignatov, V. I. & Dolgopolova, G. M. The third element effect in anodic stripping voltammetry. Anal. Chim. Acta 113, 277–285 (1980).
Chan, H., Butler, A., Falck, D. M. & Freund, M. S. Artificial neural network processing of stripping analysis responses for identifying and quantifying heavy metals in the presence of intermetallic compound formation. Anal. Chem. 69, 2373–2378 (1997).
Lastres, E. et al. Use of neural networks in solving interferences caused by formation of intermetallic compounds in anodic stripping voltammetry. Electroanalysis 9, 251–254 (1997).
Liu, N., Zhao, G. & Liu, G. Coupling square wave anodic stripping voltammetry with support vector regression to detect the concentration of lead in soil under the interference of copper accurately. Sensors 20, 6792 (2020).
Florence, T. M., Batley, G. E. & Benes, P. Chemical speciation in natural waters. Crit. Rev. Anal. Chem. 9, 219–296 (1980).
Figura, P. & McDuffie, B. Determination of labilities of soluble trace metal species in aqueous environmental samples by anodic stripping voltammetry and Chelex column and batch methods. Anal. Chem. 52, 1433–1439 (1980).
Locatelli, C. Overlapping voltammetric peaks — an analytical procedure for simultaneous determination of trace metals. Application to food and environmental matrices. Analyt. Bioanal. Chem. 381, 1073–1081 (2005).
Romanenko, S. V., Stromberg, A. G., Selivanova, E. V. & Romanenko, E. S. Resolution of the overlapping peaks in the case of linear sweep anodic stripping voltammetry via curve fitting. Chemometr. Intell. Lab. Syst. 73, 7–13 (2004).
Economou, A., Fielden, P. R. & Packham, A. J. Deconvolution of analytical peaks by means of the fast Hartley transform. Analyst 121, 1015–1018 (1996).
Turnes, G., Cladera, A., Gómez, E., Estela, J. M. & Cerdà, V. Resolution of highly overlapped differential pulse polarographic and anodic stripping voltammetric peaks by multicomponent analysis. J. Electroanal. Chem. 338, 49–60 (1992).
Górski, Ł., Kubiak, W. W. & Jakubowska, M. Independent components analysis of the overlapping voltammetric signals. Electroanalysis 28, 1470–1477 (2016).
Cladera, A. et al. Resolution of highly overlapping differential pulse anodic stripping voltammetric signals using multicomponent analysis and neural networks. Anal. Chim. Acta 350, 163–169 (1997).
Qiu, P., Ni, Y.-N. & Kokot, S. Application of artificial neural networks to the determination of pesticides by linear sweep stripping voltammetry. Chin. Chem. Lett. 24, 246–248 (2013).
Sagberg, P. & Lund, W. Trace metal analysis by anodic-stripping voltammetry effect of surface-active substances. Talanta 29, 457–460 (1982).
Wang, J. & Luo, D.-B. Effect of surface-active compounds on voltammetric stripping analysis at the mercury film electrode. Talanta 31, 703–707 (1984).
Niewiara, E., Baś, B. & Kubiak, W. W. Elimination of SAS Interferences in catalytic adsorptive stripping voltammetric determination of Cr(VI) by means of fumed silica. Electroanalysis 19, 2185–2193 (2007).
Wang, J., Deo, R. P., Thongngamdee, S. & Ogorevc, B. Effect of surface-active compounds on the stripping voltammetric response of bismuth film electrodes. Electroanalysis 13, 1153–1156 (2001).
Kefala, G., Economou, A. & Voulgaropoulos, A. A study of Nafion-coated bismuth-film electrodes for the determination of trace metals by anodic stripping voltammetry. Analyst 129, 1082–1090 (2004).
Grabarczyk, M. & Koper, A. Adsorptive stripping voltammetry of uranium: elimination of interferences from surface active substances and application to the determination in natural water samples. Anal. Methods 3, 1046–1050 (2011).
Wang, J. Decentralized electrochemical monitoring of trace metals: from disposable strips to remote electrodes. Plenary lecture. Analyst 119, 763–766 (1994).
Mahato, K. & Wang, J. Electrochemical sensors: from the bench to the skin. Sens. Actuators B Chem. 344, 130178 (2021).
Kim, J. et al. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 51, 41–45 (2015). This work presents a wearable tattoo-based sensor for zinc determination in sweat by ESA.
Malzahn, K., Windmiller, J. R., Valdés-Ramírez, G., Schöning, M. J. & Wang, J. Wearable electrochemical sensors for in situ analysis in marine environments. Analyst 136, 2912–2917 (2011).
O’Mahony, A. M., Windmiller, J. R., Samek, I. A., Bandodkar, A. J. & Wang, J. “Swipe and Scan”: integration of sampling and analysis of gunshot metal residues at screen-printed electrodes. Electrochem. Commun. 23, 52–55 (2012).
Daniele, S., Bragato, C., Antonietta Baldo, M., Wang, J. & Lu, J. The use of a remote stripping sensor for the determination of copper and mercury in the Lagoon of Venice. Analyst 125, 731–735 (2000).
Yang, Q. et al. Development of a heavy metal sensing boat for automatic analysis in natural waters utilizing anodic stripping voltammetry. ACS ES T Water 1, 2470–2476 (2021).
Wang, J. et al. Remote electrochemical sensor for trace metal contaminants. Anal. Chem. 67, 1481–1485 (1995). This work presents one of the very first remote sensors for multi-element determination of trace elements in the sea.
Zirino, A., Lieberman, S. & Clavell, C. Measurement of copper and zinc in San Diego Bay by automated anodic stripping voltammetry. Environ. Sci. Technol. 12, 73–79 (1978).
Lace, A. & Cleary, J. A review of microfluidic detection strategies for heavy metals in water. Chemosensors https://doi.org/10.3390/chemosensors9040060 (2021).
Pungjunun, K. et al. Enhanced sensitivity and separation for simultaneous determination of tin and lead using paper-based sensors combined with a portable potentiostat. Sens. Actuators B Chem. 318, 128241 (2020).
Nunez-Bajo, E., Blanco-López, M. C., Costa-García, A. & Fernández-Abedul, M. T. Electrogeneration of gold nanoparticles on porous-carbon paper-based electrodes and application to inorganic arsenic analysis in white wines by chronoamperometric stripping. Anal. Chem. 89, 6415–6423 (2017).
Kokkinos, C. & Economou, A. in Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry Vol. 7 (ed. Wandelt, K.) 261–268 (Elsevier, 2018).
Author information
Authors and Affiliations
Contributions
Introduction (A.E. and D.S.); Experimentation (C.E.B. and R.D.C.); Results (C.A. and C.P.-R.); Applications (A.B. and A.K.); Reproducibility and data deposition (C.A. and C.P.-R.); Limitations and optimizations (A.E. and D.S.); Outlook (J.W.); overview of the Primer (A.E., C.A., C.E.B., A.B., R.D.C., A.K., C.P.-R., D.S. and J.W.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Damien Arrigan, Jiri Barek, Dawei Pan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Conductivity: https://www.sciencedirect.com/topics/chemistry/conductivity
Ionic strength: https://www.sciencedirect.com/topics/chemistry/ionic-strength
Oligopeptides: https://www.sciencedirect.com/topics/chemistry/oligopeptide
Polysaccharides: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/polysaccharide
Glossary
- Pre-concentration
-
The process by which the analyte (or its compound) is deposited on the working electrode.
- Working electrode
-
An electrode at which one or more analytes undergo a redox reaction and whose response is used as an analytical signal.
- Convective mass transfer
-
A mechanism of mass transfer to the electrode based on the forced movement of solution caused by stirring, vibrations or heating.
- Voltammetry
-
An electroanalytical technique that involves scanning the potential of the working electrode versus time and measurement of the current versus potential.
- (Chrono-)Potentiometry
-
An electroanalytical technique that involves a redox reaction at the working electrode and measurement of the potential versus time.
- Stripping voltammetry
-
A two-step electrochemical measurement. The first step is to accumulate material at an electrode, whereas the second step is to measure the amount of accumulated species by voltammetry.
- Potentiometric stripping analysis
-
(PSA). An electrochemical measurement where material is accumulated at an electrode. The accumulated material is then removed either by chemical reaction or electrochemically at constant current while the electrode potential is measured.
- Potentiostat
-
An instrument used to measure electric current. It controls the potential between working and reference electrodes and measures the current between working and auxiliary electrodes.
- Reference electrode
-
An electrode with respect to which the potential of the working electrode is measured in an electrochemical cell.
- Counter electrode
-
An electrode that carries the electric current flowing through an electrochemical cell. Any electrochemical processes that occur on the surface of this electrode are not of interest.
- Non-faradaic (or capacitive) contributions
-
Refers to the current arising from an electrical double layer charging at an electrode–solution interface. Non-faradaic current is not associated with a redox reaction.
- Polarization potential window
-
A range of electrode potentials where, when compared with the current from of the investigated reaction, the electric current from the electrode or electrolyte reaction is negligible.
- Electron transfer rate
-
The rate at which electrons are exchanged between a working electrode and a redox species.
- Overpotential
-
Additional potential above the equilibrium Nernst potential value that is required for an electric current to flow through the working electrode.
- Anodic stripping voltammetry
-
(ASV). A stripping voltammetry method where electrochemical oxidation occurs during the stripping step of material accumulated at the working electrode.
- Cathodic stripping voltammetry
-
(CSV). A stripping voltammetry method where electrochemical reduction occurs during the stripping step of material accumulated at the working electrode.
- Faradaic current
-
An electric current that arises from redox reactions of electroactive species at a working electrode.
- Voltammogram
-
The representation of current versus potential.
- Constant current PSA
-
(Constant current potentiometric stripping analysis) or constant current stripping (chrono)potentiometry . An electrochemical measurement where material is accumulated at an electrode. The accumulated material is then electrochemically removed at constant current while the electrode potential is measured.
- Potentiogram
-
A representation of the differential Δt/ΔE versus potential.
- Matrix effects
-
Effects of the matrix of the sample on the analytical response.
- Matrix-matched standard solutions
-
Standard solutions prepared in a matrix similar to the matrix of the sample.
- Adsorptive stripping voltammetry
-
(AdSV). A stripping voltammetry method where pre-concentration is achieved by adsorption of the analyte or its compound.
- Underpotential deposition
-
A phenomenon of electrodeposition of a species (typically reduction of a metal cation to a solid metal) at a potential less negative than the equilibrium (Nernst) potential for the reduction of this metal (the equilibrium potential for the reduction of a metal is the potential at which the metal will deposit onto itself).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ariño, C., Banks, C.E., Bobrowski, A. et al. Electrochemical stripping analysis. Nat Rev Methods Primers 2, 62 (2022). https://doi.org/10.1038/s43586-022-00143-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-022-00143-5
This article is cited by
-
Facile and sensitive electrochemical sensing device based on carbon paste electrode for warfarin determination
Monatshefte für Chemie - Chemical Monthly (2024)
-
Are micro- and nanoelectrodes just smaller versions of regular electrodes?
Journal of Solid State Electrochemistry (2024)
-
Coupled GO–MWCNT Composite Ink for Enhanced Dispersibility and Synthesis of Screen-Printing Electrodes
Chemistry Africa (2023)
-
Wearable chemical sensors for biomarker discovery in the omics era
Nature Reviews Chemistry (2022)