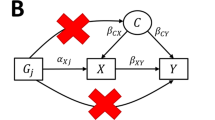
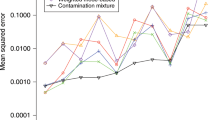
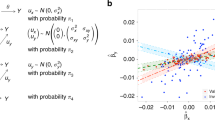
Abstract
Mendelian randomization (MR) is a term that applies to the use of genetic variation to address causal questions about how modifiable exposures influence different outcomes. The principles of MR are based on Mendel’s laws of inheritance and instrumental variable estimation methods, which enable the inference of causal effects in the presence of unobserved confounding. In this Primer, we outline the principles of MR, the instrumental variable conditions underlying MR estimation and some of the methods used for estimation. We go on to discuss how the assumptions underlying an MR study can be assessed and describe methods of estimation that are robust to certain violations of these assumptions. We give examples of a range of studies in which MR has been applied, the limitations of current methods of analysis and the outlook for MR in the future. The differences between the assumptions required for MR analysis and other forms of epidemiological studies means that MR can be used as part of a triangulation across multiple sources of evidence for causal inference.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Davey Smith, G. & Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22 (2003).
Angrist, J. D., Imbens, G. W. & Rubin, D. B. Identification of causal effects using instrumental variables. J. Am. Stat. Assoc. 91, 444–455 (1996).
Hernán, M. A. & Robins, J. M. Causal Inference: What If (Chapman & Hall/CRC, 2020).
Greenland, S. An introduction to instrumental variables for epidemiologists. Int. J. Epidemiol. 29, 722–729 (2000).
Zuccolo, L. & Holmes, M. V. Commentary: Mendelian randomization-inspired causal inference in the absence of genetic data. Int. J. Epidemiol. 46, 962–965 (2017).
Munafò, M. R., Higgins, J. P. & Davey Smith, G. Triangulating evidence through the inclusion of genetically informed designs. Cold Spring Harb. Perspect. Med. 11, a040659 (2021).
Lawlor, D. A., Tilling, K. & Davey Smith, G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45, 1866–1886 (2017).
Richmond, R. C. & Davey Smith, G. Mendelian randomization: concepts and scope. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a040501 (2022).
Davey Smith, G. & Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42 (2004).
Gupta, S. K. Intention-to-treat concept: a review. Perspect. Clin. Res. 2, 109–112 (2011).
Ellenberg, J. H. Intent-to-treat analysis versus as-treated analysis. Drug Inf. J. 30, 535–544 (1996).
Glymour, M. M. Natural experiments and instrumental variable analyses in social epidemiology. Methods Soc. Epidemiol. 1, 429 (2006).
Martens, E. P., Pestman, W. R., de Boer, A., Belitser, S. V. & Klungel, O. H. Instrumental variables: application and limitations. Epidemiology 17, 260–267 (2006).
Lousdal, M. L. An introduction to instrumental variable assumptions, validation and estimation. Emerg. Themes Epidemiol. 15, 1 (2018).
Angrist, J. D. & Krueger, A. B. Instrumental variables and the search for identification: from supply and demand to natural experiments. J. Econ. Perspect. 15, 69–85 (2001).
Rassen, J. A., Brookhart, M. A., Glynn, R. J., Mittleman, M. A. & Schneeweiss, S. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J. Clin. Epidemiol. 62, 1226–1232 (2009).
Didelez, V. & Sheehan, N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 16, 309–330 (2007).
Davey Smith, G. Capitalizing on Mendelian randomization to assess the effects of treatments. J. R. Soc. Med. 100, 432–435 (2007).
Carlson, C. S. et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am. J. Hum. Genet. 77, 64–77 (2005).
Davey Smith, G. et al. Association of C-reactive protein with blood pressure and hypertension: life course confounding and Mendelian randomization tests of causality. Arterioscler. Thromb. Vasc. Biol. 25, 1051–1056 (2005).
Morris, T. T., Heron, J., Sanderson, E., Davey Smith, G. & Tilling, K. Interpretation of Mendelian randomization using one measure of an exposure that varies over time. Preprint at medRxiv https://doi.org/10.1101/2021.11.18.21266515 (2021).
Swanson, S. A., Tiemeier, H., Ikram, M. A. & Hernán, M. A. Nature as a trialist? Deconstructing the analogy between Mendelian randomization and randomized trials. Epidemiology 28, 653–659 (2017).
Didelez, V., Meng, S. & Sheehan, N. A. Assumptions of IV methods for observational epidemiology. Statist. Sci. 25, 22–40 (2010).
Palmer, T. M. et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 21, 223–242 (2011).
Burgess, S. & Thompson, S. G. Use of allele scores as instrumental variables for Mendelian randomization. Int. J. Epidemiol. 42, 1134–1144 (2013).
Davies, N. M. et al. The many weak instruments problem and Mendelian randomization. Stat. Med. 34, 454–468 (2015).
Hernán, M. A. & Robins, J. M. Instruments for causal inference: an epidemiologist’s dream? Epidemiology 17, 360–372 (2006).
Swanson, S. A., Hernán, M. A., Miller, M., Robins, J. M. & Richardson, T. S. Partial identification of the average treatment effect using instrumental variables: review of methods for binary instruments, treatments, and outcomes. J. Am. Stat. Assoc. 113, 933–947 (2018).
Staley, J. R. & Burgess, S. Semiparametric methods for estimation of a nonlinear exposure–outcome relationship using instrumental variables with application to Mendelian randomization. Genet. Epidemiol. 41, 341–352 (2017).
Tyrrell, J. et al. Genetic predictors of participation in optional components of UK Biobank. Nat. Commun. 12, 886 (2021).
Davey Smith, G. Epigenesis for epidemiologists: does evo-devo have implications for population health research and practice? Int. J. Epidemiol. 41, 236–247 (2012).
Freeman, G., Cowling, B. J. & Schooling, C. M. Power and sample size calculations for Mendelian randomization studies using one genetic instrument. Int. J. Epidemiol. 42, 1157–1163 (2013).
Walker, V. M., Davies, N. M., Windmeijer, F., Burgess, S. & Martin, R. M. Power calculator for instrumental variable analysis in pharmacoepidemiology. Int. J. Epidemiol. 46, 1627–1632 (2017).
Burgess, S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int. J. Epidemiol. 43, 922–929 (2014).
Brion, M.-J. A., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 42, 1497–1501 (2012).
Morris, T. P., White, I. R. & Crowther, M. J. Using simulation studies to evaluate statistical methods. Stat. Med. 38, 2074–2102 (2019).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Zhao, Q., Wang, J., Spiller, W., Bowden, J. & Small, D. S. Two-sample instrumental variable analyses using heterogeneous samples. Stat. Sci. 34, 317–333 (2019).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 4, 186 (2019).
Pierce, B. L. & Burgess, S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 178, 1177–1184 (2013).
Holmes, M. V., Richardson, T. G., Ference, B. A., Davies, N. M. & Davey Smith, G. Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development. Nat. Rev. Cardiol. 18, 435–453 (2021).
Bound, J., Jaeger, D. A. & Baker, R. M. Problems with instrumental variables estimation when the correlation between the instruments and the endogenous explanatory variable is weak. J. Am. Stat. Assoc. 90, 443–450 (1995).
Burgess, S., Davies, N. M. & Thompson, S. G. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40, 597–608 (2016).
Mounier, N. & Kutalik, Z. Correction for sample overlap, winner’s curse and weak instrument bias in two-sample Mendelian Randomization. Preprint at bioRxiv https://doi.org/10.1101/2021.03.26.437168 (2021).
Angrist, J. D. & Krueger, A. B. Split-sample instrumental variables estimates of the return to schooling. J. Bus. Econ. Stat. 13, 225–235 (1995).
Fang, S., Hemani, G., Richardson, T. G., Gaunt, T. R. & Davey Smith, G. Evaluating and implementing block jackknife resampling Mendelian randomization to mitigate bias induced by overlapping samples. Preprint at medRxiv https://doi.org/10.1101/2021.12.03.21267246 (2021).
Sadreev, I. I. et al. Navigating sample overlap, winner’s curse and weak instrument bias in Mendelian randomization studies using the UK Biobank. Preprint at medRxiv https://doi.org/10.1101/2021.06.28.21259622 (2021).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362, k601 (2018).
Holmes, M. V., Ala-Korpela, M. & Davey Smith, G. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 14, 577–590 (2017).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375, n2233 (2021).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA 326, 1614–1621 (2021).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163 (2008).
Wooldridge, J. M. Econometric Analysis of Cross Section and Panel Data (MIT Press, 2010).
Cole, S. R. et al. Illustrating bias due to conditioning on a collider. Int. J. Epidemiol. 39, 417–420 (2009).
Munafò, M. R., Tilling, K., Taylor, A. E., Evans, D. M. & Davey Smith, G. Collider scope: when selection bias can substantially influence observed associations. Int. J. Epidemiol. 47, 226–235 (2018).
Hernán, M. A., Hernández-Díaz, S. & Robins, J. M. A structural approach to selection bias. Epidemiology 15, 615–625 (2004).
Staiger, D. & Stock, J. H. Instrumental variables regression with weak instruments. Report No. 0898-2937 (National Bureau of Economic Research, 1994).
Stock, J. H. & Yogo, M. Testing for weak instruments in linear IV regression. Report No. 0898-2937 (National Bureau of Economic Research, 2002).
Brumpton, B. et al. Within-family studies for Mendelian randomization: avoiding dynastic, assortative mating, and population stratification biases. Nat. Commun. 11, 1–13 (2020).
Hemani, G., Bowden, J. & Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27, R195–R208 (2018).
Davey Smith, G. & Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98 (2014).
Burgess, S., Swanson, S. A. & Labrecque, J. A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 36, 253–257 (2021).
Griffith, G. J. et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 11, 5749 (2020).
Hughes, R. A., Davies, N. M., Davey Smith, G. & Tilling, K. Selection bias when estimating average treatment effects using one-sample instrumental variable analysis. Epidemiology 30, 350–357 (2019).
Sargan, J. D. The estimation of economic relationships using instrumental variables. Econometrica 26, 393–415 (1958).
Glymour, M. M., Tchetgen Tchetgen, E. J. & Robins, J. M. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am. J. Epidemiol. 175, 332–339 (2012).
Diemer, E. W., Labrecque, J., Tiemeier, H. & Swanson, S. A. Application of the instrumental inequalities to a Mendelian randomization study with multiple proposed instruments. Epidemiology 31, 65–74 (2020).
Yang, Q., Sanderson, E., Tilling, K., Borges, M. C. & Lawlor, D. A. Exploring and mitigating potential bias when genetic instrumental variables are associated with multiple non-exposure traits in Mendelian randomization. Preprint at medRxiv https://doi.org/10.1101/19009605 (2019).
Lawlor, D. A. et al. Exploring the developmental overnutrition hypothesis using parental–offspring associations and FTO as an instrumental variable. PLoS Med. 5, e33 (2008).
Kang, H., Zhang, A., Cai, T. T. & Small, D. S. Instrumental variables estimation with some invalid instruments and its application to Mendelian randomization. J. Am. Stat. Assoc. 111, 132–144 (2016).
Windmeijer, F., Farbmacher, H., Davies, N. & Davey Smith, G. On the use of the lasso for instrumental variables estimation with some invalid instruments. J. Am. Stat. Assoc. 114, 1339–1350 (2019).
Jiang, L. et al. Constrained instruments and their application to Mendelian randomization with pleiotropy. Genet. Epidemiol. 43, 373–401 (2019).
Sanderson, E., Davey Smith, G., Windmeijer, F. & Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 48, 713–727 (2019).
Chen, L., Davey Smith, G., Harbord, R. M. & Lewis, S. J. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 5, e52 (2008).
Spiller, W., Hartwig, F. P., Sanderson, E., Davey Smith, G. & Bowden, J. Interaction-based Mendelian randomization with measured and unmeasured gene-by-covariate interactions. Preprint at medRxiv https://doi.org/10.1101/2020.07.27.20162909 (2020).
Spiller, W., Slichter, D., Bowden, J. & Davey Smith, G. Detecting and correcting for bias in Mendelian randomization analyses using gene-by-environment interactions. Int. J. Epidemiol. 48, 702–712 (2019).
Tchetgen Tchetgen, E. J., Sun, B. & Walter, S. The GENIUS approach to robust Mendelian randomization inference. Stat. Sci. 36, 443–464 (2019).
Burgess, S., Dudbridge, F. & Thompson, S. G. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat. Med. 35, 1880–1906 (2016).
Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun. 9, 224 (2018).
Hartwig, F. P., Davies, N. M., Hemani, G. & Davey Smith, G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 45, 1717–1726 (2017).
Bowden, J. et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int. J. Epidemiol. 47, 1264–1278 (2018).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Hartwig, F. P., Davey Smith, G. & Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998 (2017).
Rees, J. M., Wood, A. M., Dudbridge, F. & Burgess, S. Robust methods in Mendelian randomization via penalization of heterogeneous causal estimates. PloS ONE 14, e0222362 (2019).
Cho, Y. et al. Exploiting horizontal pleiotropy to search for causal pathways within a Mendelian randomization framework. Nat. Commun. 11, 1010 (2020).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Zhao, Q., Wang, J., Hemani, G., Bowden, J. & Small, D. S. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann. Stat. 48, 1742–1769 (2020).
Wang, J. et al. Causal inference for heritable phenotypic risk factors using heterogeneous genetic instruments. PLoS Genet. 17, e1009575 (2021).
Morrison, J., Knoblauch, N., Marcus, J. H., Stephens, M. & He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 52, 740–747 (2020).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260 (2015).
Bowden, J. & Vansteelandt, S. Mendelian randomization analysis of case-control data using structural mean models. Stat. Med. 30, 678–694 (2011).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13, e1007081 (2017).
Brown, B. C. & Knowles, D. A. Welch-weighted Egger regression reduces false positives due to correlated pleiotropy in Mendelian randomization. Am. J. Hum. Genet. 108, 2319–2335 (2021).
O’Connor, L. J. & Price, A. L. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat. Genet. 50, 1728–1734 (2018).
Elsworth, B. L. et al. The MRC IEU OpenGWAS data infrastructure. Preprint at bioRxiv https://doi.org/10.1101/2020.08.10.244293 (2020).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Snoeckx, R. L. et al. GJB2 mutations and degree of hearing loss: a multicenter study. Am. J. Hum. Genet. 77, 945–957 (2005).
Hoffmann, T. J. et al. A large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet. 12, e1006371 (2016).
Lambert, J.-C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
Burgess, S., Dudbridge, F. & Thompson, S. G. Re: “Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects”. Am. J. Epidemiol. 181, 290–291 (2015).
Zuber, V., Colijn, J. M., Klaver, C. & Burgess, S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat. Commun. 11, 29 (2020).
Sanderson, E. Multivariable Mendelian randomization and mediation. Cold Spring Harb. Perspect. Med. 11, a038984 (2020).
Carter, A. R. et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur. J. Epidemiol. 36, 465–478 (2021).
Relton, C. L. & Davey Smith, G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int. J. Epidemiol. 41, 161–176 (2012).
Burgess, S., Daniel, R. M., Butterworth, A. S., Thompson, S. G. & Consortium, E.-I. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int. J. Epidemiol. 44, 484–495 (2015).
Burgess, S., Davies, N. M. & Thompson, S. G. Instrumental variable analysis with a nonlinear exposure–outcome relationship. Epidemiology 25, 877 (2014).
Sun, Y.-Q. et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses. BMJ 364, l1042 (2019).
North, T.-L. et al. Using genetic instruments to estimate interactions in Mendelian randomization studies. Epidemiology 30, e33–e35 (2019).
Rees, J., Foley, C. N. & Burgess, S. Factorial Mendelian randomization: using genetic variants to assess interactions. Int. J. Epidemiol. 49, 1147–1158 (2019).
Plagnol, V., Smyth, D. J., Todd, J. A. & Clayton, D. G. Statistical independence of the colocalized association signals for type 1 diabetes and RPS26 gene expression on chromosome 12q13. Biostatistics 10, 327–334 (2009).
Wallace, C. Statistical testing of shared genetic control for potentially related traits. Genet. Epidemiol. 37, 802–813 (2013).
Pavlides, J. M. W. et al. Predicting gene targets from integrative analyses of summary data from GWAS and eQTL studies for 28 human complex traits. Genome Med. 8, 84–84 (2016).
Hormozdiari, F. et al. Colocalization of GWAS and eQTL signals detects target genes. Am. J. Hum. Genet. 99, 1245–1260 (2016).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 16, e1008720 (2020).
Marmot, M. & Brunner, E. Alcohol and cardiovascular disease: the status of the U shaped curve. BMJ 303, 565–568 (1991).
Corrao, G., Rubbiati, L., Bagnardi, V., Zambon, A. & Poikolainen, K. Alcohol and coronary heart disease: a meta-analysis. Addiction 95, 1505–1523 (2000).
Mukamal, K. J. & Rimm, E. B. Alcohol’s effects on the risk for coronary heart disease. Alcohol. Res. Health 25, 255–261 (2001).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03169530 (2019).
Dyer, O. $100m alcohol study is cancelled amid pro-industry “bias”. BMJ 361, k2689 (2018).
Mitchell, G., Lesch, M. & McCambridge, J. Alcohol industry involvement in the moderate alcohol and cardiovascular health trial. Am. J. Public Health 110, 485–488 (2020).
National Institutes of Health. NIH to end funding for Moderate Alcohol and Cardiovascular Health trial. National Institutes of Health https://www.nih.gov/news-events/news-releases/nih-end-funding-moderate-alcohol-cardiovascular-health-trial (2018).
Wild, C. in World Cancer Report 2014 (eds Wild, C. P. & Stewart, B. W.) (World Health Organization, 2014).
Secretan, B. et al. A review of human carcinogens — Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 10, 1033–1034 (2009).
Lawlor, D. A. et al. Exploring causal associations between alcohol and coronary heart disease risk factors: findings from a Mendelian randomization study in the Copenhagen General Population Study. Eur. Heart J. 34, 2519–2528 (2013).
Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349, g4164 (2014).
Silverwood, R. J. et al. Testing for non-linear causal effects using a binary genotype in a Mendelian randomization study: application to alcohol and cardiovascular traits. Int. J. Epidemiol. 43, 1781–1790 (2014).
Millwood, I. Y. et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393, 1831–1842 (2019).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015).
Miller, G. & Miller, N. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 305, 16–19 (1975).
Castelli, W. P. et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 55, 767–772 (1977)
Emerging Risk Factors Collaboration et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302, 1993–2000 (2009).
Davey Smith, G. & Phillips, A. N. Correlation without a cause: an epidemiological odyssey. Int. J. Epidemiol. 49, 4–14 (2020).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380, 572–580 (2012).
Do, R. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45, 1345–1352 (2013).
Holmes, M. V. et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 36, 539–550 (2015).
Holmes, M. V. & Davey Smith, G. REVEALing the effect of CETP inhibition in cardiovascular disease. Nat. Rev. Cardiol. 14, 635–636 (2017).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Riaz, H. et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 26, 533–543 (2019).
Richardson, T. G., Sanderson, E., Elsworth, B., Tilling, K. & Davey Smith, G. Use of genetic variation to separate the effects of early and later life adiposity on disease risk: Mendelian randomisation study. BMJ 369, m1203 (2020).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Schooling, C. M. Selection bias in population-representative studies? A commentary on Deaton and Cartwright. Soc. Sci. Med. 210, 70 (2018).
Dixon, P., Davey Smith, G., von Hinke, S., Davies, N. M. & Hollingworth, W. Estimating marginal healthcare costs using genetic variants as instrumental variables: Mendelian randomization in economic evaluation. PharmacoEconomics 34, 1075–1086 (2016).
Dixon, P., Hollingworth, W., Harrison, S., Davies, N. M. & Davey Smith, G. Mendelian randomization analysis of the causal effect of adiposity on hospital costs. J. Health Econ. 70, 102300 (2020).
Xu, Z. M. & Burgess, S. Polygenic modelling of treatment effect heterogeneity. Genet. Epidemiol. 44, 868–879 (2020).
Holmes, M. V. Human genetics and drug development. N. Engl. J. Med. 380, 1076–1079 (2019).
Kyriacou, D. N. & Lewis, R. J. Confounding by indication in clinical research. JAMA 316, 1818–1819 (2016).
Schmidt, A. F. et al. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 11, 3255 (2020).
Schmidt, A. F., Hingorani, A. D. & Finan, C. Human genomics and drug development. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a039230 (2021).
Munafò, M. R. et al. A manifesto for reproducible science. Nat. Hum. Behav. 1, 0021 (2017).
Munafò, M. R. & Davey Smith, G. Robust research needs many lines of evidence. Nature 553, 399–401 (2018).
Davies, N. M., Dickson, M., Davey Smith, G., van den Berg, G. J. & Windmeijer, F. The causal effects of education on health outcomes in the UK Biobank. Nat. Hum. Behav. 2, 117–125 (2018).
Sanderson, E., Davey Smith, G., Bowden, J. & Munafò, M. R. Mendelian randomisation analysis of the effect of educational attainment and cognitive ability on smoking behaviour. Nat. Commun. 10, 2949 (2019).
Davies, N. M. et al. Multivariable two-sample Mendelian randomization estimates of the effects of intelligence and education on health. eLife 8, e43990 (2019).
Tillmann, T. et al. Education and coronary heart disease: mendelian randomisation study. BMJ 358, j3542 (2017).
Davies, N. M., Dickson, M., Davey Smith, G., Windmeijer, F. & van den Berg, G. J. The causal effects of education on adult health, mortality and income: evidence from Mendelian randomization and the raising of the school leaving age. Preprint at SSRN https://doi.org/10.2139/ssrn.3390179 (2019).
Baldwin, J., Pingault, J.-B., Schoeler, T., Sallis, H. M. & Munafo, M. R. Protecting against researcher bias in secondary data analysis: challenges and solutions. Eur. J. Epidemiol. 37, 1–10 (2022).
Sallis, H. Triangulation protocol; intergenerational effects of parental substance use on child substance use and mental health outcomes. Preprint at https://osf.io/s6jv4/ (2021).
Hartwig, F. P., Davies, N. M. & Davey Smith, G. Bias in Mendelian randomization due to assortative mating. Genet. Epidemiol. 42, 608–620 (2018).
Brumpton, B. et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat. Commun. 11, 3519 (2020).
Morris, T. T., Davies, N. M., Hemani, G. & Davey Smith, G. Population phenomena inflate genetic associations of complex social traits. Sci. Adv. 6, eaay0328 (2020).
Minică, C. C., Boomsma, D. I., Dolan, C. V., de Geus, E. & Neale, M. C. Empirical comparisons of multiple Mendelian randomization approaches in the presence of assortative mating. Int. J. Epidemiol. 49, 1185–1193 (2020).
Davies, N. M. et al. Within family Mendelian randomization studies. Hum. Mol. Genet. 28, R170–R179 (2019).
Minică, C. C., Dolan, C. V., Boomsma, D. I., de Geus, E. & Neale, M. C. Extending causality tests with genetic instruments: an integration of Mendelian randomization with the classical twin design. Behav. Genet. 48, 337–349 (2018).
Howe, L. J. et al. Within-sibship genome-wide association analyses decrease bias in estimates of direct genetic effects. Nat. Genet. (in the press).
Taylor, A. E. et al. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 47, 1207–1216 (2018).
Fry, A. et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017).
Pirastu, N. et al. Genetic analyses identify widespread sex-differential participation bias. Nat. Genet. 53, 663–671 (2021).
Smit, R. A., Trompet, S., Dekkers, O. M., Jukema, J. W. & le Cessie, S. Survival bias in Mendelian randomization studies: a threat to causal inference. Epidemiology 30, 813 (2019).
Schooling, C. M. et al. Use of multivariable Mendelian randomization to address biases due to competing risk before recruitment. Front. Genet. 11, 610852 (2020).
Vansteelandt, S., Dukes, O. & Martinussen, T. Survivor bias in Mendelian randomization analysis. Biostatistics 19, 426–443 (2017).
Hernán, M. A. Invited commentary: selection bias without colliders. Am. J. Epidemiol. 185, 1048–1050 (2017).
Mahmoud, O., Dudbridge, F., Davey Smith, G., Munafo, M. & Tilling, K. Slope-Hunter: a robust method for index-event bias correction in genome-wide association studies of subsequent traits. Nat. Commun. (in the press).
Waddington, C. H. Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 (1942).
Debat, V. & David, P. Mapping phenotypes: canalization, plasticity and developmental stability. Trends Ecol. Evol. 16, 555–561 (2001).
Kitami, T. & Nadeau, J. H. Biochemical networking contributes more to genetic buffering in human and mouse metabolic pathways than does gene duplication. Nat. Genet. 32, 191–194 (2002).
Gu, Z. et al. Role of duplicate genes in genetic robustness against null mutations. Nature 421, 63–66 (2003).
Hemani, G. et al. Automating Mendelian randomization through machine learning to construct a putative causal map of the human phenome. Preprint at bioRxiv https://doi.org/10.1101/173682 (2017).
Ioannidis, J. P. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 94, 485–514 (2016).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51, 584–591 (2019).
Paternoster, L., Tilling, K. & Davey Smith, G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet. 13, e1006944 (2017).
Zhou, W. et al. Causal relationships between body mass index, smoking and lung cancer: univariable and multivariable Mendelian randomization. Int. J. Cancer 148, 1077–1086 (2021).
Lee, J. C. et al. Genome-wide association study identifies distinct genetic contributions to prognosis and susceptibility in Crohn’s disease. Nat. Genet. 49, 262–268 (2017).
Kim, Y.-I. Role of folate in colon cancer development and progression. J. Nutr. 133, 3731S–3739S (2003).
Davey Smith, G., Paternoster, L. & Relton, C. When will Mendelian randomization become relevant for clinical practice and public health? JAMA 317, 589–591 (2017).
Ye, T., Shao, J. & Kang, H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann. Stat. 49, 2079–2100 (2021).
Bowden, J. et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 48, 728–742 (2018).
Wang, S. & Kang, H. Weak-instrument robust tests in two-sample summary-data Mendelian randomization. Biometrics https://doi.org/10.1111/biom.13524 (2021).
Minelli, C. et al. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol. 50, 1651–1659 (2021).
Burgess, S., Foley, C. N., Allara, E., Staley, J. R. & Howson, J. M. M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 11, 376 (2020).
Foley, C. N., Mason, A. M., Kirk, P. D. W. & Burgess, S. MR-Clust: clustering of genetic variants in Mendelian randomization with similar causal estimates. Bioinformatics 37, 531–541 (2020).
Berzuini, C., Guo, H., Burgess, S. & Bernardinelli, L. A Bayesian approach to Mendelian randomization with multiple pleiotropic variants. Biostatistics 21, 86–101 (2018).
Xu, S., Fung, W. K. & Liu, Z. MRCIP: a robust Mendelian randomization method accounting for correlated and idiosyncratic pleiotropy. Brief. Bioinform. 22, bbab019 (2021).
Qi, G. & Chatterjee, N. Mendelian randomization analysis using mixture models for robust and efficient estimation of causal effects. Nat. Commun. 10, 1941 (2019).
Cheng, Q. et al. MR-LDP: a two-sample Mendelian randomization for GWAS summary statistics accounting for linkage disequilibrium and horizontal pleiotropy. NAR Genom. Bioinform 2, lqaa028 (2020).
Zhu, X., Li, X., Xu, R. & Wang, T. An iterative approach to detect pleiotropy and perform Mendelian randomization analysis using GWAS summary statistics. Bioinformatics 37, 1390–1400 (2020).
Grant, A. J. & Burgess, S. An efficient and robust approach to Mendelian randomization with measured pleiotropic effects in a high-dimensional setting. Biostatistics https://doi.org/10.1093/biostatistics/kxaa045 (2020).
Iong, D., Zhao, Q. & Chen, Y. A latent mixture model for heterogeneous causal mechanisms in mendelian randomization. Preprint at https://arxiv.org/abs/2007.06476 (2020).
van der Graaf, A. et al. Mendelian randomization while jointly modeling cis genetics identifies causal relationships between gene expression and lipids. Nat. Commun. 11, 4930 (2020).
Jiang, L., Xu, S., Mancuso, N., Newcombe, P. J. & Conti, D. V. A hierarchical approach using marginal summary statistics for multiple intermediates in a Mendelian randomization or transcriptome analysis. Am. J. Epidemiol. 190, 1148–1158 (2021).
DiPrete, T. A., Burik, C. A. P. & Koellinger, P. D. Genetic instrumental variable regression: explaining socioeconomic and health outcomes in nonexperimental data. Proc. Natl Acad. Sci. USA 115, E4970–E4979 (2018).
Howey, R., Shin, S.-Y., Relton, C., Davey Smith, G. & Cordell, H. J. Bayesian network analysis incorporating genetic anchors complements conventional Mendelian randomization approaches for exploratory analysis of causal relationships in complex data. PLoS Genet. 16, e1008198 (2020).
Schmidt, A. F. & Dudbridge, F. Mendelian randomization with Egger pleiotropy correction and weakly informative Bayesian priors. Int. J. Epidemiol. 47, 1217–1228 (2017).
Bucur, I. G., Claassen, T. & Heskes, T. Inferring the direction of a causal link and estimating its effect via a Bayesian Mendelian randomization approach. Stat. Methods Med. Res. 29, 1081–1111 (2019).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur. J. Epidemiol. 35, 99–111 (2020).
Davey Smith, G. et al. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 4, 1985–1992 (2007).
Pearl, J. Causality (Cambridge Univ. Press, 2009).
Keele, L., Zhao, Q., Kelz, R. R. & Small, D. Falsification tests for instrumental variable designs with an application to tendency to operate. Med. Care 57, 167–171 (2019).
Brookhart, M. A., Rassen, J. A. & Schneeweiss, S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol. Drug Saf. 19, 537–554 (2010).
Burgess, S. & Labrecque, J. A. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur. J. Epidemiol. 33, 947–952 (2018).
Wang, L. & Tchetgen Tchetgen, E. Bounded, efficient and multiply robust estimation of average treatment effects using instrumental variables. J. R. Stat. Soc. Ser. B 80, 531–550 (2018).
Mills, H. L. et al. Detecting heterogeneity of intervention effects using analysis and meta-analysis of differences in variance between arms of a trial. Epidemiology 32, 846–854 (2021).
West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford Univ. Press, 2003).
Zuckerkandl, E. & Villet, R. Concentration-affinity equivalence in gene regulation: convergence of genetic and environmental effects. Proc. Natl Acad. Sci. USA 85, 4784–4788 (1988).
Ebrahim, S. & Davey Smith, G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum. Genet. 123, 15–33 (2008).
Hill, W. D. et al. Molecular genetic contributions to social deprivation and household income in UK Biobank. Curr. Biol. 26, 3083–3089 (2016).
Labrecque, J. A. & Swanson, S. A. Interpretation and potential biases of mendelian randomization estimates with time-varying exposures. Am. J. Epidemiol. 188, 231–238 (2018).
Sanderson, E., Richardson, T. G., Morris, T. T., Tilling, K. & Davey Smith, G. Estimation of causal effects of a time-varying exposure at multiple time points through Multivariable Mendelian randomization. Preprint at medRxiv https://doi.org/10.1101/2022.01.04.22268740 (2022).
Cardon, L. R. & Palmer, L. J. Population stratification and spurious allelic association. Lancet 361, 598–604 (2003).
Loh, P.-R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284 (2015).
Zhou, W. et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat. Genet. 50, 1335–1341 (2018).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Lawson, D. J. et al. Is population structure in the genetic biobank era irrelevant, a challenge, or an opportunity? Hum. Genet. 139, 23–41 (2020).
Haworth, S. et al. Apparent latent structure within the UK Biobank sample has implications for epidemiological analysis. Nat. Commun. 10, 1–9 (2019).
Howe, L. J. et al. Genetic evidence for assortative mating on alcohol consumption in the UK Biobank. Nat. Commun. 10, 5039 (2019).
Nordsletten, A. E. et al. Patterns of nonrandom mating within and across 11 major psychiatric disorders. JAMA Psychiat. 73, 354–361 (2016).
Bochud, M., Chiolero, A., Elston, R. C. & Paccaud, F. A cautionary note on the use of Mendelian randomization to infer causation in observational epidemiology. Int. J. Epidemiol. 37, 414–416 (2008).
Acknowledgements
E.S., M.R.M., T.P. and G.D.S. are members of the UK Medical Research Council (MRC) Integrative Epidemiology unit, which is funded by the MRC (MC_UU_00011/1, MC_UU_00011/3 and MC_UU_00011/7) and the University of Bristol. M.M.G. is supported by the National Institutes of Health/National Institute on Aging (NIH/NIA) grant R01AG057869. M.V.H. works in a unit that receives funding from the MRC and is supported by a British Heart Foundation Intermediate Clinical Research Fellowship (FS/18/23/33512) and the National Institute for Health Research Oxford Biomedical Research Centre. H.K. is supported by the National Science Foundation grant DMS-1811414. C.W. is funded by the MRC (MC UU 00002/4, MC UU 00002/13) and the Wellcome Trust (WT107881).
Author information
Authors and Affiliations
Contributions
Introduction (E.S.); Experimentation (E.S., M.M.G. and T.P); Results (E.S., M.M.G., T.P. and C.W); Applications (E.S. and M.V.H.); Reproducibility and data deposition (M.R.M.); Limitations and optimizations (E.S.); Outlook (G.D.S.); Overview of the Primer (E.S., H.K., J.M., C.M.S., Q.Z. and G.D.S.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Methods Primers thanks Marianne Benn, Frida Emanuelsson, Sarah Gagliano Taliun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ivonesamplemr: https://github.com/remlapmot/ivonesamplemr
MendelianRandomization: https://cran.r-project.org/package=MendelianRandomization
MR dictionary: https://mr-dictionary.mrcieu.ac.uk/
mrrobust: https://github.com/remlapmot/mrrobust
OneSampleMR: https://remlapmot.github.io/OneSampleMR/
STROBE-MR: https://www.strobe-mr.org/
The OpenGWAS project: https://gwas.mrcieu.ac.uk/
TwoSampleMR: https://github.com/MRCIEU/TwoSampleMR
UK Biobank: https://www.ukbiobank.ac.uk/
Supplementary information
Glossary
- Instrumental variable
-
(IV). A variable associated with an exposure that is not associated with the outcome through any other pathway.
- Natural experiment
-
Natural experiments are variation in any exposures or risk factors that occurred by chance in the population without conscious or deliberate intervention from investigators or scientists.
- Confounder
-
A trait that influences both the exposure and outcome of interest.
- First-stage F statistic
-
Test statistic used to test the strength of association between the instrument(s) and the exposure in an instrumental variable estimation.
- Linkage disequilibrium
-
Correlation between genetic variants located closely together on the genome.
- Vertical pleiotropy
-
The phenomenon of a genetic variant associated with multiple phenotypes on the same pathway.
- Horizontal pleiotropy
-
The phenomenon of a genetic variant associated with multiple phenotypes on different pathways.
- Bidirectional relationship
-
Where an effect acts in both directions between a pair of traits so that changing one will change the other.
- Collider bias
-
Bias occurring owing to conditioning on a variable that is dependent on both the exposure and outcome or is dependent on causes of the exposure and outcome.
Rights and permissions
About this article
Cite this article
Sanderson, E., Glymour, M.M., Holmes, M.V. et al. Mendelian randomization. Nat Rev Methods Primers 2, 6 (2022). https://doi.org/10.1038/s43586-021-00092-5
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-021-00092-5
This article is cited by
-
Mendelian randomization study supports positive bidirectional causal relationships between genetically predicted insomnia symptom and liability to benign prostatic hyperplasia
BMC Urology (2024)
-
MRSamePopTest: introducing a simple falsification test for the two-sample mendelian randomisation ‘same population’ assumption
BMC Research Notes (2024)
-
Genetic evidence of the causal relationship between chronic liver diseases and musculoskeletal disorders
Journal of Translational Medicine (2024)
-
Evaluation of the causal effects of blood metabolites on irritable bowel syndrome: Mendelian randomization
BMC Gastroenterology (2024)
-
Proteomic associations with forced expiratory volume: a Mendelian randomisation study
Respiratory Research (2024)