Abstract
The future vulnerability of low-lying atoll nations is inextricably linked to the production of carbonate sediments by organisms living in their adjacent marine environments. Seagrass meadows are commonly found adjacent to reef islands, but their role as sources of reef island-building sediments has been overlooked. Here, we combine field, satellite and sedimentological data to quantify rates of sediment production by seagrass epibionts in a reef island sediment supply context. Total seagrass epibiont sediment production at our study site (Huvadhoo Atoll, Maldives) was 853,000 ± 90,000 kg CaCO3 yr−1 over an area of 1.1 km2. Of this total sediment production, 541,000 ± 23,000 kg CaCO3 yr−1 was estimated to be suitable to contribute to reef island building (sand-sized, post-agitation). Our findings highlight a valuable ecosystem service provided by tropical seagrass meadows as important potential sources of reef island-building sediment. This study, therefore, presents a compelling geomorphic argument for seagrass conservation.
Similar content being viewed by others
Introduction
Low-lying coral reef islands are among the world’s most vulnerable environments to the impacts of climate change. With elevations of <3 m above mean sea level, flooding and sea-level rise are projected to render reef islands uninhabitable by the end of the century1. The fate of reef islands is of global importance as they provide the only habitable land within atoll nations, which support a collective human population of >700,0002. Reef islands are, in part, vulnerable to environmental change because they are formed entirely of carbonate (CaCO3) sediments produced by organisms living in their adjacent marine environments. Consequently, shifts in the adjacent marine ecology drive changes in the rates and types (grain size, as well as constituent and mineralogical composition) of carbonate sediment production, with potential adverse consequences for reef island physical resilience to sea-level rise3. Accurate predictions of the physical trajectories of atoll nations and reef islands are, therefore, contingent upon a comprehensive understanding of marine carbonate sediment production.
While there is increasing understanding of the production of reef island-building sediments associated with some organisms (e.g., parrotfish4,5,6), understanding – or acknowledgement – of the capacity of seagrass meadows to produce reef island-building sediments is near non-existent. Yet, these habitats are recognised carbonate sources7,8,9,10,11,12 and are widespread around many low-lying reef islands. A key mechanism of seagrass sediment production is associated with leaf epibiota; carbonate-producing organisms that live on seagrass leaves, such as benthic foraminifera, molluscs, serpulids and crustose coralline algae (CCA, Fig. 1). These calcareous epibionts directly contribute to the sediment reservoir after either being dislodged from the seagrass leaf, or when the seagrass leaf they are living on dies10. Mud-sized sediment (<0.063 mm; descriptive nomenclature of Udden-Wentworth is used throughout) has previously been highlighted as a common component of the seagrass epibiont sediment production pathway8,9,11. However, it is sand- (0.063–2 mm) and gravel- (>2 mm) sized sediments that are most suitable for reef island building as they have a greater residence time on reef platforms than finer, mud-sized sediments, which are typically winnowed off reef3,5,13 or lost in solution14. For example, five Maldivian islands were found to be comprised of ~98% sand- and gravel-sized sediment15,16. However, to the best of our knowledge, the potential for seagrass meadows (via epibiont carbonate production) to produce sand-sized sediments remains unquantified in reef island sediment supply contexts. This knowledge gap exists despite recognition of the importance of sand-sized sediment production for the development and future physical resilience of reef islands globally3. Addressing this knowledge gap is especially relevant in regions such as the Maldives where seagrass is actively removed by the tourist industry for aesthetic reasons17. Such data are important to ensure that the impacts of seagrass removal can be effectively assessed and considered in decision-making processes (e.g., development consenting processes and marine spatial planning).
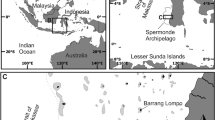
Here, we investigate the potential role of seagrass meadows as sources of sediments that are suitable for reef island building (i.e., sand-sized). To quantify rates of epibiont sediment production, we combined detailed field data, high-resolution satellite imagery and sedimentological analyses from a 1.1 km2 study site off the oceanward coast of Faathihutta island, Huvadhoo Atoll, Maldives (Fig. 2). This field site was selected as a representative seagrass meadow as 74% of the 27 km2 area of seagrass on Huvadhoo Atoll is located on the oceanward sides of atoll rim platforms18. Specifically, we calculated the: (1) rates of seagrass epibiotic sediment production; and (2) proportion of epibiotic sediment produced that was of an appropriate grade (sand-sized) to contribute to reef island building.
Locations of a the Maldives within the Indian Ocean (imagery: ESRI, 202061); b Huvadhoo Atoll within the southern Maldives; c the study site within Huvadhoo Atoll; and d the site area (in red) and ground truth points within each zone (PlanetScope satellite imagery62). e Classification of seagrass zones.
Results and discussion
Site zonation
Our sampling design was structured by dividing the site into three zones, one of which was split into two sub-zones, on the basis of seagrass leaf density, percentage cover and species composition (Table 1 and Fig. 2): (1) sub-Zone 1a was characterised by the highest leaf densities (1,937 ± 98 per m2) and the predominance of Thalassodendron ciliatum (91 ± 3%) growing in approximately circular-shaped patches (Fig. 2f); (2) sub-Zone 1b had the lowest seagrass cover (22 ± 13%) and was also T. ciliatum dominated (100 ± 0%); (3) Zone 2 was a mixed species zone (69 ± 4% T. ciliatum and 31 ± 4% Thalassia hemprichii), which was observed to grow with more continuous seagrass coverage than that found in the other zones (Fig. 2f); and (4) Zone 3 was dominated by T. hemprichii (86 ± 4%) with lower seagrass percentage cover (24 ± 3%). An unsupervised classification of seagrass zones was generated from PlanetScope satellite imagery (spatial resolution = 3 m2) with an overall accuracy of 98% (Fig. 2e).
Sediment production rates
Sediment production associated with seagrass epibionts was 762,000 ± 90,000 kg CaCO3 per year over the site area of 1.1 km2. Total sand-sized (post- 21 days of continuous agitation in water) sediment production across the site was 482,000 ± 23,000 kg CaCO3 per year. This rate of sand-sized sediment production is equivalent to the approximate volume of the adjacent island of Fhaatihutta over an ~18 year period (island area = 5773 m2; elevation = 0.81 m).
The rates of seagrass epibiont sediment production ranged from 0.22 ± 0.13 kg CaCO3 m−2 per year in sub-Zone 1b to 0.86 ± 0.21 kg CaCO3 m−2 per year in Zone 3 (Table 1; Fig. 3). Differences in sediment production rates between zones reflect differences in seagrass leaf densities, species abundance, the mass of epibionts on leaves, and species-specific turnover data. The higher rates of sediment production in Zone 3 were primarily a function of the faster turnover of T. ciliatum, which was dominant in this zone. In this context, the mass of sediment per seagrass leaf ranged from 0.05 ± 0.01 g CaCO3 (Zones 1a and 3) to 0.07 ± 0.01 g CaCO3 (Zone 2; Table 1; Fig. 3). The mass of epibionts was significantly higher on T. ciliatum than T. hemprichii leaves (F1,32 = 5.00; P = 0.03; two-way ANOVA). No significant interaction was found between seagrass zone and species (F3,32 = 0.37; P = 0.78; two-way ANOVA).
a The mass of sediment (CaCO3) per seagrass leaf associated with Thalassodendron ciliatum and Thalassia hemprichii within each zone, as determined through loss after acidification analyses. The box limits are the lower (Q1) and upper (Q3) quartiles, and the median is represented by the centre lines. Whiskers represent the minimum and maximum values before the lower and upper fences, respectively. Diamonds represent the mean. Rates of b total and c sand-sized sediment production associated with each species within each seagrass zone. Error bars represent the cumulative errors6.
Sediment mass per seagrass leaf was significantly higher in Zone 2 (0.07 ± 0.01 g CaCO3; Table 1) than in any other zone (F3,32 = 4.21; P = 0.01; two-way ANOVA). Zone 2 was characterised by continuous seagrass cover, in contrast to the more circular seagrass patches found in Zone 1 and the lower percentage cover of Zone 3 (Fig. 2f and Table 1). One possible explanation for the higher masses of sediment per seagrass leaf in Zone 2 is that continuous seagrass cover is associated with high photosynthetic activity, which would increase seawater pH and, in turn, calcification rates19,20,21. An alternative hypothesis is that areas of more continuous seagrass cover, such as found in Zone 2, are more effective at baffling wave energy22 and that such lower wave energies may enable larger masses of epibionts to accumulate on seagrass leaves. Further study is required to validate this hypothesis, but if correct, this function has implications for seagrass conservation and management as seagrass removal inherently decreases the continuity of seagrass cover and increases habitat fragmentation. The impacts of seagrass removal upon sediment production may, therefore, extend beyond the removal zone by increasing seagrass habitat fragmentation, which may reduce the sediment production capacity of seagrasses adjacent to the removal zone.
Reef island-building sediment production
From an island sediment supply perspective, it is important to quantify the proportion of epibiotic sediment production that is of an appropriate size fraction (sand-sized) to contribute to island building. As reef island building necessitates sediment transport by waves from marine sites of production to reef island shorelines, sediment samples were subjected to 21 days of continuous agitation in water to simulate wave action. Epibiotic carbonate grain size distributions were analysed pre- and post- 21 days of continuous agitation12. Pre-agitation, mean grain size across all samples was 589 ± 504 µm, with a sand-sized fraction (appropriate for reef island building) of 78 ± 13% (Table 1 and Fig. 4). Post-agitation, mean grain size was 237 ± 51 µm, with a sand-sized fraction of 61 ± 10%. Notably, pre-agitation grain size distributions were highly variable between samples (standard deviation = 504 µm; Fig. 4). In contrast, post-agitation grain size distributions were strikingly similar (standard deviation = 51 µm; Fig. 4), suggesting that the sediments had reached, or were approaching, their end-point grain sizes3,23, though ongoing gradual abrasion and dissolution would also be likely in marine environments. No significant differences in mean grain size were found between species or zones either pre- (P = 0.08 and 0.12, respectively; Kruskal–Wallis) or post- (P = 0.91 and 0.82, respectively; Kruskal–Wallis) agitation.
These rates of sediment production and the high-proportion of post-agitation sand-sized sediment demonstrate an important functional role for seagrass as generators of carbonate sediments that are suitable for island building. The potential contribution of seagrass epibionts (benthic foraminifera, CCA, molluscs)10 to reef island sediment reservoirs is evident by the composition of the upper sedimentary horizons of three reef islands located on the reef platform immediately west of our field site, which comprise 1.4 ± 0.5% benthic foraminifera, 12.8 ± 1.1% CCA, and 5.2 ± 0.8% molluscs15. However, our estimates of sediment production are likely to be underestimates of actual epibiotic sediment production because: (1) sediment production in seagrass meadows is not limited only to epibionts, rather seagrasses provide habitats for a range of sediment-producing infaunal and epifaunal organisms, including foraminifera24, gastropods25, and urchins26; (2) seagrasses provide a nursery ground for parrotfish, a key producer of reef island-building sediments in the Maldives4,5,6 whereby, depending on species, a proportion of sediment generated as a by-product of parrotfish grazing is in the sand-sized fraction (mostly coral and CCA)4,5,6; (3) instrumental limitations necessitated the removal of gravel-sized sediments (>2 mm; e.g. foraminifera, gastropods) from volumetric grain size analysis; and (4) we used the longest published turnover rates to derive conservative estimates of sediment production rates (Supplementary Table 1 and 2). Interestingly, based on the shortest published turnover rates, sediment production rates at our site would have ranged from 1.0 ± 0.1 kg CaCO3 m−2 per year in Zone 1b to 2.3 ± 0.1 kg CaCO3 m−2 per year in Zone 1a. In the absence of measured site-specific species turnover rates and given the sensitivity of sediment production calculations to turnover data, we opted to use the most conservative values to avoid any overestimations in calculations.
Inevitably, not all sediment production within seagrass meadows will contribute to reef island building. Rather, sediment may either remain stored in the marine environment, potentially trapped by seagrass leaves27, or be transported off the reef platform by waves28. Nonetheless, our estimates indicate that seagrass meadows can generate substantial amounts of geomorphically important sediment within reef island systems. Where sediment remains trapped by seagrass leaves within the marine environment, this additional sediment will contribute to their vertical accretion, and thus to a shallowing of the seabed29. It is possible that such a shallowing of the marine environment may serve to protect reef islands from coastal erosion, particularly in the context of sea-level rise, as it would reduce the proportion of incident wave energy that is able to propagate across reef platform surfaces and reach island shorelines30,31,32.
Our study focused on an oceanward seagrass meadow as the most typical location of seagrass meadows within Huvadhoo Atoll. However, epibiotic sediment production from lagoonward seagrass meadows may also represent an important source of reef island-building sediments. Established reef island chronologies from eastern and southwestern atoll rim platforms on Huvadhoo Atoll have highlighted that islands have laterally accreted toward atoll lagoons. This lagoonward accretion was facilitated by sand-sized sediments derived from lagoonward marine environments, which likely contributed to island building16. In the Maldives, it is likely that lagoonward seagrass meadows on eastern atoll rim platforms are particularly important sources of reef island-building sediments. As wind is predominantly westerly, wind-generated waves build towards the east of the atolls due to the long fetch distances across atoll lagoons, meaning lagoonward wave energy is at a maximum on eastern atoll rims30,32. Indeed, lagoonward island accretion was found to be most pronounced on the eastern rim of Huvadhoo Atoll16.
Previous reef island sediment budgets have highlighted that narrow outer reef zones can play a disproportionately important role (relative to their size) in platform-scale sediment budgets4,33. For example, on an atoll rim platform in Lhaviyani Atoll, northern-central Maldives, sediment production rates were highest (up to 5.18 kg CaCO3 m−2 per year) in an outer patch reef zone that occupied only 6.5% of the reef platform area33. These rates are markedly higher (per m2) than those reported in the present study for seagrass meadows in an atoll rim reef setting (up to 0.86 kg CaCO3 m−2 per year in Zone 3). However, where present, seagrass meadows are not constrained to narrow outer reef zones, but rather their habitat has the potential to extend across and occupy large areas of reef platform lagoons and reef flats. In the Maldives, seagrass cover is 174% larger (118 km2) than the area covered by reef crests (68 km2)18. Similarly, in other atoll nations, seagrass cover (32.5 km2) is 146% larger than that of reef crests (22.3 km2) in Kiribati; and seagrass cover (0.72 km2) is 157% greater than that of reef crests (0.46 km2) in the Marshall Islands18. Hence, seagrass meadows have the potential to make notable contributions to platform- and national-scale reef sediment budgets.
Implications
Our results highlight the capacity of seagrass epibionts to produce substantial quantities of carbonate sediment that is geomorphically important for reef island building. Blue carbon storage34 and habitat provision35 are typically considered among the primary seagrass ecosystem services. However, we highlight that the capacity of seagrass meadows to produce substantive volumes of sediments suitable for reef island building represents an important additional seagrass ecosystem service. This ecosystem service is of global significance as it adds to the natural capital value36 of seagrass by increasing the physical resilience of coastlines adjacent to seagrass meadows to erosion. This ecosystem service provides a compelling argument for the need to halt the direct (e.g. removal, dredging, coastal construction) and indirect (e.g. pollution, threats associated with climate change)37 loss of seagrass from both marine environments17 and beach deposits38.
Upscaling our findings to the national scale suggests that Maldivian seagrass meadows are producing some 81,183 ± 18,421 tonnes CaCO3 per year, of which 51,353 ± 12,118 tonnes CaCO3 per year (63%) is predicted to be sand-sized material. These values were estimated by multiplying the national seagrass area18 (118 km2) by the average epibiotic seagrass sediment production rate of 688 ± 156 g m−2 CaCO3 per year, of which an average of 435 ± 103 g m−2 CaCO3 per year was sand-sized. However, these estimates do not account for spatial variability in environmental conditions (which can impact the mass of epibiotic communities39,40), seagrass leaf densities, and seagrass species composition. At both national and international scales, such data are typically lacking, but are is required to meaningfully upscale epibiotic sediment production rates in a robust manner. Such data could be used to provide evidence to inform accurate natural capital assessments for consideration in decision-making processes in coastal development and marine conservation41. Furthermore, in any location with epibiont encrusted seagrasses, associated carbonate sediment production will have the capacity to contribute to building and maintaining adjacent shorelines, and should thus be considered in the context of coastal sediment budgets.
Seagrass meadows face a range of threats that could compromise their capacity for epibiotic sediment production. Local threats include direct removal, coastal pollution and coastal construction37. Superimposed upon local threats are those associated with climate change, for example: (1) increased storm frequency and/or severity, which could provide a short-term increase in the delivery of sediment to reef island shorelines42,43, but may compromise the capacity of seagrass meadows to recover44; (2) increasing sea temperatures may cause a poleward range shift in tropical seagrasses, which could be detrimental to equatorial seagrasses45; (3) sea-level rise may reduce light required for photosynthesis46; and (4) ocean acidification is likely to have negative impacts upon calcareous epibionts47,48,49, which would reduce capacity for sediment production. However, seagrasses may buffer ocean acidification by removing CO2 from the water column through photosynthetic activity, which can increase local seawater pH19,20,50. Whilst seagrasses face numerous climate change threats, at present, the decline in coral health as a result of bleaching appears to be more pronounced in the Maldives51,52 than any declines in seagrass. There is a paucity of seagrass monitoring data from the Maldives, though one report suggests a 20% increase in seagrass area was found between 2019 and 2020 on a reef platform in Laamu Atoll53.
For atoll nations, sediment production is particularly important because they are formed entirely of sediments derived from carbonate-producing organisms in their adjacent marine environments. In the absence of sediment generation, island physical resilience is contingent upon the adjustment of only a finite volume of sediment. Seagrass sediment production may therefore enhance the physical resilience of reef islands to the impacts of climate change, which may become increasingly important given threats to coral health54. For example, Maldivian reef islands are predominantly comprised of coral sediments (~77%)15,16, which could be problematic given declines in coral health within the region51,52.
The role of seagrass as a source of reef island-building sediment thus ought to be considered in coastal management plans and consenting processes, particularly as marine construction activities (e.g., harbours, jetties, breakwaters, water bungalows, dredging, land reclamation) often occur in lagoonal areas of potential seagrass habitat55. Lagoonal construction activities ought to avoid both negative impacts to seagrass meadows, and obstructing seagrass-to-island sediment transport pathways. Indeed, seagrass conservation ought to be considered as a nature-based solution that may increase shoreline resilience to coastal erosion and reef island physical resilience to sea-level rise.
Conclusion
We present the first, to the best of our knowledge, estimates of sediment production by seagrass epibionts in a reef island sediment supply context. Our results demonstrate that seagrass meadows can produce substantive quantities of carbonate sediment that is suitable for reef island building. Such sediment production is of importance as it may increase the future physical resilience of reef islands with adjacent seagrass meadows to the impacts of climate change. The production of reef island-building sediments, and associated shoreline maintenance, represents a highly valuable ecosystem service provided by seagrass meadows that has received little acknowledgement to-date. Whilst arguments for seagrass conservation typically focus on their significance for blue carbon storage and supporting biodiversity, our data highlight that sediment production adds to the natural capital value of seagrass and represents a compelling geomorphic argument for seagrass conservation.
Methods
Detailed surveys and sampling were conducted to quantify the rates of epibiotic carbonate production within a 1.1 km2 seagrass meadow on the oceanward reef flat adjacent to Faathihutta island, Huvadhoo Atoll, Maldives (Fig. 2). Huvadhoo Atoll experiences a semidiurnal microtidal regime (range = ~1 m). Two seagrass species were present in this site, T. ciliatum56 and T. hemprichii57. To structure sampling design, the site was divided into zones based on ecological parameters (seagrass species composition, leaf densities, and seagrass percentage cover) determined through snorkel surveys and remote sensing analyses (Unmanned Aerial Vehicle and Planet satellite imagery)4. An unsupervised classification was performed on Planet satellite imagery acquired on 8 April 2019 (spatial resolution = 3 m2) to calculate the area of each seagrass zone. Ground truth data were obtained from each zone (04-05/2019; n = 180) to validate the classification.
Seagrass data were collected at sampling points (n = 100) along four oceanward-lagoonward transects (04-05/2019; Fig. 2d). At each sampling point, three 0.25 × 0.25 m quadrats were randomly placed (n = 300) and within each we quantified (1) seagrass percentage cover of each species, and (2) the number of seagrass leaves (in total, 27,528 seagrass leaves were manually counted). The proportions of each species were then multiplied by leaf counts to estimate species-level seagrass leaf densities.
The weight loss after acidification method was used to estimate the mass of carbonate epibionts (g CaCO3) produced per seagrass leaf per species within each zone7,58. At five random sampling points within each zone, ten representative mature seagrass leaves of each species were selected and individually analysed (n = 400, 50 leaves analysed per species in each zone). To determine natural weight loss of leaves during acidification (e.g. due to CaCO3 leaf content11), 10 unencrusted leaves were also analysed from each species within each zone. Seagrass epibiont calcium carbonate produced was calculated as7,58:
where, Eprod = epibiont calcium carbonate production (g CaCO3 per leaf), A = weight of encrusted (g) dry leaves, B = weight of acid treated leaves (g), C = weight loss from unencrusted acid treated leaves (%).
Species-level rates of CaCO3 production within each zone (kg CaCO3 m−2 per year−1) were calculated by multiplying loss after acidification data by the average species-level leaf densities (per m2) per zone, and species-specific annual leaf turnover rates. As published species-level turnover data were found to be highly variable (Supplementary Tables 1 and 2), we used the longest available turnover rates to derive the most conservative estimates of sediment production rates. Specifically, the turnover values used were 94 days (3.9 crops per year) for T. ciliatum and 30 days (12.2 crops per year) for T. hemprichii.
To determine the proportion of sand-sized sediment production, bulk seagrass samples of each species were collected at three randomly selected sites within each zone (n = 24). Following collection, epibionts were hand scraped from leaves using a scalpel, and soaked in a dilute solution (5%) of sodium hypochlorite for 24 hours to remove organic matter59. Sodium hypochlorite is known to have no discernable impact upon carbonate grain structures59. Epibionts were then analysed using a Malvern Mastersizer 2000 laser particle sizer to determine their grain size distributions. As reef island building necessitates sediment transport by waves from marine sites of production to reef island shorelines, the grain size of scraped samples is likely to overestimate the proportion of sand-sized sediment as grain size may decrease during transport. To simulate sediment transport and associated wave action, each sample was recovered from the Mastersizer and subjected to 21 days of continuous agitation in water using a IKA HS 501 horizontal shaker (set to 106 oscillations per minute). This approach for quantifying sediment breakdown was adapted from Perry et al.12 but instead used a horizontal shaker. A 21-day timeframe was selected following Perry et al.’s approach12, though it is likely that sediments would continue to breakdown over longer periods than 21 days in the marine environment. However, rates of marine sediment breakdown would likely be substantially higher in our lab experiment than in the marine environment as a high number of oscillations (106 per minute) was selected to ensure that grains were continually in motion and to maximise sediment breakdown within the timeframe. Following agitation, sample grain size distributions were re-analysed using the Malvern Mastersizer 2000.
Epibiont sediment production rates were multiplied by the average percentage of (post-agitation) sand-sized sediment associated with each species in each zone to determine sand-sized sediment production rates. Finally, species-level sediment production rates calculated for each zone were added and then multiplied by zone areas (as calculated from the Planet-derived classification) to calculate total and sand-sized sediment production. Standard rules for error propagation were used to calculate the cumulative errors6.
Statistical tests were performed within R software60. Assumptions for the tests were assessed in all instances. Where assumptions were met, ANOVA tests were used to determine whether there were significant differences between groups of data. In several instances, the data were not normally distributed and so a Kruskal–Wallis test was used.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The datasets generated during and/or analysed during the current study are available on the Figshare repository with the identifier: https://doi.org/10.6084/m9.figshare.21751685.
References
Storlazzi, C. D. et al. Most atolls will be uninhabitable by the mid-21st century because of sea-level rise exacerbating wave-driven flooding. Sci. Adv. 4, eaap9741 (2018).
World Bank. World Bank Group. World Bank https://www.worldbank.org/en/home (2021).
Perry, C. T. et al. Implications of reef ecosystem change for the stability and maintenance of coral reef islands. Glob. Change Biol. 17, 3679–3696 (2011).
Perry, C. T., Kench, P. S., O’Leary, M. J., Morgan, K. M. & Januchowski-Hartley, F. Linking reef ecology to island building: parrotfish identified as major producers of island-building sediment in the Maldives. Geology 43, 503–506 (2015).
Morgan, K. M. & Kench, P. S. Parrotfish erosion underpins reef growth, sand talus development and island building in the Maldives. Sediment. Geol. 341, 50–57 (2016).
Yarlett, R. T., Perry, C. T., Wilson, R. W. & Harborne, A. R. Inter-habitat variability in parrotfish bioerosion rates and grazing pressure on an Indian Ocean Reef Platform. Diversity 12, 381 (2020).
Nelsen, J. E. & Ginsburg, R. N. Calcium carbonate production by epibionts on Thalassia in Florida Bay. J. Sediment. Res. 56, 622–628 (1986).
Land, L. S. Carbonate mud; production by epibiont growth on Thalassia testudinum. J. Sediment. Res. 40, 1361–1363 (1970).
Patriquin, D. G. Carbonate mud production by epibionts on Thalassia; an estimate based on leaf growth rate data. J. Sediment. Res. 42, 687–689 (1972).
Corlett, H. & Jones, B. Epiphyte communities on Thalassia testudinum from Grand Cayman, British West Indies: Their composition, structure, and contribution to lagoonal sediments. Sediment. Geol. 194, 245–262 (2007).
Enríquez, S. & Schubert, N. Direct contribution of the seagrass Thalassia testudinum to lime mud production. Nat. Commun. 5, 1–12 (2014).
Perry, C. T., Salter, M. A., Morgan, K. M. & Harborne, A. R. Census estimates of algal and epiphytic carbonate production highlight tropical seagrass meadows as sediment production hotspots. Front. Mar. Sci. 6, 120 (2019).
Kench, P. S. A currents of removal approach for interpreting carbonate sedimentary processes. Mar. Geol. 145, 197–223 (1998).
Andersson, A. J. & Gledhill, D. Ocean acidification and coral reefs: effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar. Sci. 5, 321–348 (2013).
East, H. K., Perry, C. T., Kench, P. S. & Liang, Y. Atoll-scale comparisons of the sedimentary structure of coral reef rim islands, Huvadhu Atoll, Maldives. J. Coast. Res. 75, 577–581 (2016).
East, H. K., Perry, C. T., Kench, P. S., Liang, Y. & Gulliver, P. Coral reef island initiation and development under higher than present sea levels. Geophys. Res. Lett. 45, 11265–11274 (2018).
BLUE Marine Foundation. ProtectMaldivesSeagrass. Maldives Resilient Reefs http://www.maldivesresilientreefs.com/seagrass/ (2021).
Allen Coral Atlas. Imagery, Maps And Monitoring Of The World’s Tropical Coral Reefs https://doi.org/10.5281/zenodo.3833242. (2022).
Frankignoulle, M. & Distèche, A. CO2 chemistry in the water column above a Posidonia seagrass bed and related air-sea exchanges. Oceanol. Acta 7, 209–219 (1984).
Semesi, I. S., Beer, S. & Björk, M. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 382, 41–47 (2009).
Unsworth, R. K. F., Collier, C. J., Henderson, G. M. & McKenzie, L. J. Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environ. Res. Lett. 7, 024026 (2012).
Fonseca, M. S. & Cahalan, J. A. A preliminary evaluation of wave attenuation by four species of seagrass. Estuar. Coast. Shelf Sci. 35, 565–576 (1992).
Scoffin, T. P. Taphonomy of coral reefs: a review. Coral Reefs 11, 57–77 (1992).
Mateu-Vicens, G., Box, A., Deudero, S. & Rodríguez, B. Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp. J. Foraminifer. Res. 40, 134–147 (2010).
Rueda, J. L., Salas, C., Urra, J. & Marina, P. Herbivory on Zostera marina by the gastropod Smaragdia viridis. Aquat. Bot. 90, 253–260 (2009).
Alcoverro, T. & Mariani, S. Patterns of fish and sea urchin grazing on tropical Indo‐Pacific seagrass beds. Ecography 27, 361–365 (2004).
de Boer, W. F. Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia 591, 5–24 (2007).
Morgan, K. M. & Kench, P. S. A detrital sediment budget of a Maldivian reef platform. Geomorphology 222, 122–131 (2014).
Alemu I, J. B., Puah, J. Y. & Friess, D. A. Shallow surface elevation changes in two tropical seagrass meadows. Estuar. Coast. Shelf Sci. 273, 107875 (2022).
Brander, R. W., Kench, P. S. & Hart, D. Spatial and temporal variations in wave characteristics across a reef platform, Warraber Island, Torres Strait, Australia. Mar. Geol. 207, 169–184 (2004).
Kench, P. S., Brander, R. W., Parnell, K. E. & O’Callaghan, J. M. Seasonal variations in wave characteristics around a coral reef island, South Maalhosmadulu atoll, Maldives. Mar. Geol. 262, 116–129 (2009).
East, H. K., Perry, C. T., Beetham, E. P., Kench, P. S. & Liang, Y. Modelling reef hydrodynamics and sediment mobility under sea level rise in atoll reef island systems. Glob. Planet. Change 192, 103196 (2020).
Perry, C. T., Morgan, K. M. & Yarlett, R. T. Reef habitat type and spatial extent as interacting controls on platform-scale carbonate budgets. Front. Mar. Sci. 4, 185 (2017).
Fourqurean, J. W. et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509 (2012).
Unsworth, R. K. F., Nordlund, L. M. & Cullen‐Unsworth, L. C. Seagrass meadows support global fisheries production. Conserv. Lett. 12, e12566 (2019).
Costanza, R. et al. The value of the world’s ecosystem services and natural capital. Nature 387, 253–260 (1997).
Waycott, M. et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 106, 12377–12381 (2009).
Ruju, A. et al. Ecosystem services of reed and seagrass debris on a urban Mediterranean beach (Poetto, Italy). Estuar. Coast. Shelf Sci. 271, 107862 (2022).
Martínez-Crego, B., Prado, P., Alcoverro, T. & Romero, J. Composition of epiphytic leaf community of Posidonia oceanica as a tool for environmental biomonitoring. Estuar. Coast. Shelf Sci. 88, 199–208 (2010).
Gullström, M., Baden, S. & Lindegarth, M. Spatial patterns and environmental correlates in leaf-associated epifaunal assemblages of temperate seagrass (Zostera marina) meadows. Mar. Biol. 159, 413–425 (2012).
Bateman, I. J. & Mace, G. M. The natural capital framework for sustainably efficient and equitable decision making. Nat. Sustain. 3, 776–783 (2020).
Leonard, L. A., Hine, A. C., Luther, M. E., Stumpf, R. P. & Wright, E. E. Sediment transport processes in a west-central florida open marine marsh tidal creek; the role of tides and extra-tropical storms. Estuar. Coast. Shelf Sci. 41, 225–248 (1995).
Remy, F., Michel, L. N., Mascart, T., De Troch, M. & Lepoint, G. Trophic ecology of macrofauna inhabiting seagrass litter accumulations is related to the pulses of dead leaves. Estuar. Coast. Shelf Sci. 252, 107300 (2021).
Oprandi, A. et al. Effects of a severe storm on seagrass meadows. Sci. Total Environ. 748, 141373 (2020).
Hyndes, G. A. et al. Accelerating tropicalization and the transformation of temperate seagrass meadows. BioScience 66, 938–948 (2016).
Grantham, H. S. et al. Ecosystem-based adaptation in marine ecosystems of tropical Oceania in response to climate change. Pac. Conserv. Biol. 17, 241–258 (2011).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (2013).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009).
Cox, T. E. et al. Effects of ocean acidification on Posidonia oceanica epiphytic community and shoot productivity. J. Ecol. 103, 1594–1609 (2015).
Bergstrom, E., Silva, J., Martins, C. & Horta, P. Seagrass can mitigate negative ocean acidification effects on calcifying algae. Sci. Rep. 9, 1932 (2019).
Perry, C. T. & Morgan, K. M. Post-bleaching coral community change on southern Maldivian reefs: is there potential for rapid recovery? Coral Reefs 36, 1189–1194 (2017).
Ibrahim, N. et al. Status of coral bleaching in the Maldives 2016. 47 (2017).
Roe, P. Six Senses Laamu Seagrass Monitoring 2020. 1–9 (2020).
Oppenheimer, M. et al. Chapter 4: Sea Level Rise and Implications for Low-Lying Islands, Coasts and Communities — Special Report on the Ocean and Cryosphere in a Changing Climate. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (ed. Pörtner, H.-O.) (IPCC, 2019).
Duvat, V. K. E. & Magnan, A. K. Rapid human-driven undermining of atoll island capacity to adjust to ocean climate-related pressures. Sci. Rep. 9, 15129 (2019).
den Hartog, C. The Seagrasses of the World (North-Holland Publishing Company, 1970).
Ascherson, P. F. A. Die geographische Verbreitung der Seegräser. vol. 17 (1871).
Bosence, D. Biogenic carbonate production in florida bay. Bull. Mar. Sci. 44, 419–433 (1989).
Gaffey, S. J. & Bronnimann, C. E. Effects of bleaching on organic and mineral phases in biogenic carbonates: RESEARCH METHOD PAPER. J. Sediment. Res. 63, 752–754 (1993).
R Core Team. R: A Language And Environment For Statistical Computing,Vienna, Austria, URL https://www.R-project.org/ (2022).
Esri. “World Imagery” [basemap]. December 17, 2020. https://www.arcgis.com/home/item.html?id=829656a742924e189e63bfc9fde75225 (2022).
Planet Team. Planet Application Program Interface: In Space for Life on Earth. https://api.planet.com (2017).
Acknowledgements
We thank the Small Island Research Group and LaMer for facilitating fieldwork. Many thanks to the government of the Maldives for permission to undertake this research under the Ministry of Fisheries, Marine Resources and Agriculture permit number (OTHR)30-D/INDIV/2019/102. This work was funded by a British Society for Geomorphology Early Career Researcher Grant to H.K. East.
Author information
Authors and Affiliations
Contributions
H.K.E. conceived the study. H.K.E., J.A.J. and C.T.P. designed the study approach. H.K.E., J.A.J., A.M. and H.Z. undertook field data collection. G.F. undertook the laboratory work with supervision from H.K.E.; M.F. undertook the literature review of seagrass turnover data. H.K.E. and J.A.J. analysed the data. H.K.E., J.A.J. and C.T.P. prepared the manuscript. All authors provided contributions to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Ulisse Cardini, Mats Björk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Clare Davis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
East, H.K., Johnson, J.A., Perry, C.T. et al. Seagrass meadows are important sources of reef island-building sediment. Commun Earth Environ 4, 33 (2023). https://doi.org/10.1038/s43247-023-00675-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-023-00675-y
This article is cited by
-
Rapid seagrass meadow expansion in an Indian Ocean bright spot
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.