Abstract
Measurements of life-history traits can reflect an organism’s response to environment. In wave-dominated rocky intertidal ecosystems, obtaining in-situ measurements of key grazing invertebrates are constrained by extreme conditions. Recent research demonstrates mollusc shells to be high-resolution sea-surface temperature proxies, as well as archival growth records. However, no prior molluscan climate proxy or life-history reconstruction has been demonstrated for the tropical rocky intertidal environment—a zone influenced by warmer waters, mixed tides, trade-wind patterns, and wave-action. Here, we show near-daily, spatiotemporal oxygen isotope signatures from the tropical rocky intertidal environment by coupling secondary ion mass spectrometry analysis of oxygen isotopes with the sclerochronology of an endemic Hawaiian intertidal limpet Cellana sandwicensis, that is a significant biocultural resource harvested for consumption. We also develop a method for reliable interpretation of seasonal growth patterns and longevity in limpets. This study provides a robust approach to explore tropical intertidal climatology and molluscan life-history.
Similar content being viewed by others
Introduction
Understanding the phenotypic plasticity of marine species to environmental change is crucial to understand the dynamics of coastal populations. Recent responses across multiple marine taxa to extreme sea surface temperature (SST) anomalies highlight the need for intertidal research focusing on thermal tolerance and habitat shifts1,2. For rocky intertidal ecology, however, monitoring climate responses in-situ presents significant challenges under adverse tide-, wave-, and wind-exposed conditions; and sub-annually resolved oceanographic climate proxies have only recently been identified for this semi-terrestrial environment3,4,5,6,7. Similar to other oceanographic climate proxies (i.e., coral skeletons, fish otoliths, foraminifera), most mollusc shell are precipitated in isotopic equilibrium with seawater8. In these accretionary hard-tissues, oxygen isotope ratio (δ18O) measurements can be aligned with physiochemical features to infer seasonality with more negative values reflecting warmer climate and more positive values reflecting cooler climate3,7,9,10,11,12.
To date, robust reconstruction of seasonal to millennial sea-surface temperature (SST) from mollusc shells are numerous in mid-to-high latitudes—where δ18O is strongly correlated with seawater temperature9,13,14,15. In contrast, SST proxies in low latitudes are limited to subtidal organisms (primarily corals), as hydrological processes (i.e., rainfall, estuarian mixing) influencing sea surface salinity (SSS) can confound drivers of δ18O16. Therefore, while corals are established long-term proxies of mean annual SST17,18,19,20, a high-resolution proxy for tropical intertidal climate is missing entirely.
Mollusc limpet shells are an excellent candidate for tropical intertidal climate proxy records due to their archeological preservation, wide distribution, and sequential growth (Twadle et al., 2016). With absolute temporal alignment of growth, ecologists can interpret species’ responses to physiological (i.e., ontogeny, reproduction) and environmental (i.e., extreme climate, tide) factors21,22,23,24,25.
Within the Hawaiian Archipelago, endemic intertidal limpets (Cellana spp.) are a significant biocultural resource declining in abundance and experiencing contracting population distributions26. Due to complex and extreme rocky intertidal conditions, research on growth patterns of Cellana is limited27,28.
To reliably reconstruct Hawaiian limpet growth patterns, we applied measurements of oxygen isotopes from secondary ion mass spectrometry (SIMS) from shell line and increment features formed during growth cessation (with represented time periods in parentheses): major growth line (annual cycle), minor growth lines (lunar cycle), and minor growth increments (tidal cycle). For these culturally and commercially important molluscan shellfish, resolving growth patterns and longevity has critical implications for aquaculture, conservation, and fisheries. Here we reconstructed the life-history of the yellowfoot limpet Cellana sandwicensis from three shells, two modern and one historical, by (1) investigating oxygen isotope variation in the tropical intertidal environment using near-daily spatial scale SIMS analysis, and (2) determining seasonal growth and longevity. This study provides a robust approach to explore tropical intertidal temperature climatology and molluscan life-history.
Results
Within each of the three shells of C. sandwicensis, five carbonate mineralogical microstructure layers were revealed by SEM and Raman microscopy in accordance with MacClintock, C. (1967). With reference to the myostracum or muscle attachment layer (M), we observed one interior layer—aragonitic, radial crossed-lamella layer (M−1)—and two exterior layers—aragonitic, concentric crossed-lamellar layer (M + 1) and calcite, concentric crossed-foliated layer (M + 2), the latter being suitable for isotope measurements (Fig. 1). The carbonate polymorph was unidentifiable for the shell’s outermost layer—a radial crossed-foliated layer (M + 3).
Shells were sectioned from anterior to posterior end. a White lines represent parallel cuts for thick-section preparation from historical specimen BPBM 250851-200492. b Cross-sections show the true direction of growth for limpets. c SEM exposed shell microstructures for area denoted in b. Oxygen isotope measurements were performed along the direction of growth in the crossed-foliated, calcite layer M + 2.
The observed VPDB corrected δ18Ocalcite values from SIMS of three shells ranged from −5.04‰ to −7.74‰ (modern specimen CW1), −4.38‰ to −7.83‰ (modern specimen CW2), and −0.57‰ to −5.02‰ (historical specimen BPBM) (see Fig. 2, Supplementary Fig. 5, Supplementary Table 1, Supplementary Table 2). Across four annual isotope cycles, the BPBM oxygen isotope profile follows a sinusoidal pattern indicative of seasonal changes in the shell records of C. sandwicensis. Based on analytical precision (2 standard deviations) and measurement precision (2 standard errors), the maximum uncertainty for δ18Ocalcite was 0.51 ‰.
A shell cross-section and the associated oxygen isotope profile (δ18O) of a Hawaiian limpet (C. sandwicensis), reported in permille (‰) relative to the international VPDB standard, were measured sequentially along the growth axis—starting at the shell margin. This pattern in the δ18O profile of the historical shell (BPBM—green line) reflects the recorded seasonality in intertidal SST. The positive δ18O measurements (red squares) were taken along and correspond with major bands (red circles) in the shell cross-section. Error bars represent measurement error 2 sigma (2σ), which reflects both precision (2 standard error) and reproducibility (2 standard deviation).
The correlation between measured and calculated δ18Ocalcite were strong and significant for both modern specimens (R2 = 0.71; p < 0.0001 and R2 = 0.69; p < 0.0001 for CW1 and CW2, respectively) (Fig. 3). In the linear regression model, slopes were 0.39 and 0.37 for CW1 and CW2, respectively. The variance in δ18Ocalcite is likely a combined signal of δ18Oseawater and SST.
We modeled reconstructed SST profiles for the historical shell across a range of ecologically relevant salinity values and found an effect of evaporation on δ18Ocalcite (Fig. 4). The reconstructed SST values changed by 0.84 ± 0.04 °C psu−1. Temperature thresholds (Tmin and Tmax) were most biologically relevant at salinity of 42 psu (see Supplementary Methods, and Supplementary Figure 3). At 42 psu, SST ranged from 15.5 °C to 38.0 °C and averaged 27.1 ± 7.38 °C.
Reconstructed sea surface temperature (SST) from historical specimen (BMBP) were modeled for ten different sea surface salinities (SSS) assuming that δ18Ocalcite (per mil, VPDB) standard is precipitated in equilibrium with δ18Oseawater (per mil, VPDB) for Cellana sandwicensis. Based on the known range of modern SST along the Hawaiian rocky intertidal ecosystem, 15 °C to 40 °C, the most biologically relevant SSS appears to be 42 psu. The sample number refers to sequentially and isotopically measured points in the direction of growth along the specimen.
The shell growth lines varied in physical appearance, which were correlated with environmental changes in the SST of the Hawaiian rocky intertidal (Fig. 5). Minor line appearance and periodicity was variable, and likely influenced by wave exposure (thick line). Micro lines were consistent and followed daily lunar cycles. We classified micro lines into two semi-lunidian increments (solid and dashed lines). During full and new moon phases, spring tides were recorded as prominent, narrow daily micro lines. During first and last quarter moon phases, neap tides were recorded as faint, wide micro lines. The major-growth lines (annual) always corresponded to the most positive δ18O measurements or lowest temperature record (Fig. 2). A major-growth line was also present in specimen BPBM—in alignment with the most negative δ18O measurement. However, this major band was only observed once throughout the multi-annual shell record and void in specimens CW1 and CW2. These major-growth lines were pronounced and spanned the width of M + 2 layer, usually intersecting visible notches on the external surface of the shell. Minor-growth lines were observed in varying intervals of micro-growth increments (circalunidian); and micro-growth increments were subdivided by observed micro-growth lines (circatidal). The micro-growth increment widths ranged from 6.32 µm to 61.74 µm, but were typically less pronounced and wider for neap tides in comparison to spring tides, which were very pronounced and narrow. The average micro-growth increment widths for each specimen were 22.54 µm (CW1), 20.67 µm (CW2), and 13.69 µm (BPBM). The estimated age for modern and historical specimens was 2 years (CW1 and CW2) and 5 years (BPBM), respectively. These age estimates were based on the number of annual isotope cycles, and early ontogenetic growth recorded in the apex region (beyond the last isotope measurement).
The back-calculated shell length measurements from calendar year 2017 were used to interpret sub-monthly growth rates of C. sandwicensis for the two modern specimens (see supplementary Table 4). Initial SL during this period were 20.75 mm (CW1) and 22.89 mm (CW2), respectively. Daily growth rates (DGSL) ranged from −83 µm to 588 µm, and averaged 140 ± 93 µm (CW1) and 98 ± 95 µm (CW2), respectively. Across monthly observations, DGSL was found to be highest in May for CW1 and January for CW2, respectively; and across seasons, DGSL was highest in Spring for CW1 and Winter for CW2, respectively. Total monthly growth ranged from 0 mm to 5.39 mm and were significantly correlated with both growth frequency (R2 = 0.81; p < 0.0001; CW1 and R2 = 0.67; p = 0.0005; CW2) and DGSL (R2 = 0.61; p = 0.0017; CW1 and R2 = 0.89; p < 0.0001; CW2), respectively. The total growth across calendar year 2017 for CW1 and CW2 was 19.96 mm and 15.01 mm, respectively. Modern limpets exhibited zero growth in both December and March, which lies in the primary spawning period. These limpets also exhibited zero growth during Summer (June-July for CW2 and August for CW1), which lies in the secondary spawning period. Furthermore, interrupted growth coincided with the most extreme oxygen isotope measurements.
For the historical specimen, the annual growth rate steadily decreased from 23.22 mm in the first year to 4.50 mm in the last year and averaged 13.83 ± 7.77 mm across a total of five isotope cycles. The average daily growth rate for BPBM was 81.78 ± 120.55 µm (see Supplementary Table 3); and the maximum daily growth recorded was 605 µm. Monthly growth rates were not determined.
To compare shell growth rates, we modeled each of the three shells using a von-Bertalanffy growth function (VBGF). For the best fit VBGF model to pooled data from all three shells, parameter estimates were \({L}_{{{{{{\rm{\infty }}}}}}}\,\)= 75.233 (±2.681 SE) and K = 0.0371 (±0.0021 SE) with \({t}_{0}\) constrained to represent an initial length of post-larval settlement of 0.254 mm observed for C. sandwicensis (Fig. 6);29,30,31. Modeled shell length at one year is 27.2 mm, two years is 44.5 mm, three years is 55.5 mm, four years is 62.6 mm, and five years is 67.1 mm. No differences in growth model parameter estimates between pairwise comparisons of individual shells were found for all χ2 statistics (p > 0.05) from likelihood ratio tests.
Discussion
We used secondary ion mass spectrometry (SIMS) analysis to achieve sub-weekly to daily spatial scale resolution in the oxygen isotope profile of a tropical rocky intertidal marine mollusc (Cellana sandwicensis). Isotope measurements were extracted from an area of 15 µm2 across the shell growth axis, which accounts for one to three growth days of any shell. To the best of our knowledge, this methodology represents the highest attainable spatio-temporal resolution for paleoecology of a tropical intertidal limpet.
The linear relationship between calculated and measured δ18O validates the use of molluscan shell records for seasonal interpretations of growth and life-history. At this time, however, we cannot conclude biogenic calcite to be in equilibrium with seawater. Future studies should focus on validating this mollusc limpet as a proxy for sea surface temperature by comparing δ18Ocalcite and δ18Oseawater using both conventional gas source mass spectrometry (GSMS) and SIMS analytical methodologies. This would contribute to the ongoing work to refine the interpretation of δ18O measured by SIMS in biogenic carbonate32,33
There was a seasonal shift in the relationship between calculated and measured δ18O for modern specimens. We do not, however, totally discount the possibility that modifications in seawater chemistry of the extrapallial fluid are influencing calcification in C. sandwicensis (Langer et al. 2018); and vital effects (i.e., physiology, metabolism) are known to interfere with oxygen isotope fractionation that cause discrepancies in molluscan climate proxies23.
To calculate oxygen isotope equilibrium fractionation (Eq. 1), we chose to apply an average salinity value for the study site; and because SSS and δ18Oseawater are highly correlated, our resulting changes in δ18Ocalcite are driven by seasonal temperature fluctuations.
Given δ18Oseawater was not measured directly, our maximum uncertainty of 0.51‰ may be slightly underestimated as surface δ18Oseawater is somewhat influenced by wet and dry seasons. We did not observe any major anomalies in the shell records. Rainfall events were previously thought to challenge interpretation of oxygen isotope profiles in tropical environments. We suggest that precipitation at both the modern shell (Ka’alawai) and the historical (unknown) sites occurs infrequently and the distance between isotope measurement locations were too far to detect these alterations in carbonate chemistry. Moreover, aside from rainfall and groundwater discharge, microhabitats within the same littoral zone vary tremendously with respect to evaporation, and are perhaps contributing to intraspecies variation in carbonate chemistry. For instance, limpets inhabiting aerially exposed rock features may result in a wider δ18Ocalcite range than sub-surface limpets7,21.
We reconstructed the past marine environment for an archival specimen (BMBP) by modeling proxy temperature regimes across a range of predicted salinities. We observe proxy temperature to approach a biologically relevant range at 42 psu. Given that our archival shell was recently collected (not part of a midden), the isotopic results suggest that both the δ18O and SSS in the Hawaiian intertidal were possibly elevated by evaporation. At a salinity of 42 psu, we interpret this historical specimen’s environment to be more aerially exposed compared to that of modern specimens, where extreme upper- and lower-threshold temperatures are likely a result of solar irradiance and evaporative cooling, respectively.
To address the precision of our inferences, we must consider that variability in the δ18Oseawater –SSS relationship may introduce error up to 1.5 PSU in the Tropical Pacific34. Fortunately, when considering this relationship in the recent geologic time scale, there has been only marginal fluctuations34. Henceforth, our current methodology should also support sound paleoecology research using tropical archeological middens dating as far back as early Polynesian settlement (AD 800) in the Hawaiian Islands. We discuss in further detail the thermal thresholds to growth, patterns in shell growth, and longevity of C. sandwicensis interpreted from the study.
Thermal threshold
The observed seasonal minimum and maximum δ18Ocalcite vary by shell record, and thresholds appear to change across time and space. Historical specimen BPBM recorded the widest δ18O range, and according to the model, did not grow below 15.5 °C or above 38.0 °C. Based on Hawaiian rocky intertidal substratum temperatures (15 °C to 40 °C), we understand this to reflect true thermal range for yellowfoot limpet35. Comparatively, modern specimens from Ka’alawai recorded a less extreme range of δ18O values, which may suggest differences between the individuals’ positioning within the intertidal environment.
Growth patterns
Tropical limpets are exposed to mixed, semi-diurnal tides, which plays a role in growth line formation. Tide patterns allowed us to temporally align growth features around anchors (δ18O min/max) similar to previous studies on temperate limpet species3,7. These patterns, however, were often interrupted at random causing inconsistent daily band widths. We attribute these inconsistencies to be influenced by waves, which dominate Hawaiian rocky intertidal shorelines36.
Besides tidally influenced growth patterns, modern specimens exhibited a decrease in daily growth rate prior to complete stoppage in somatic growth in primary and secondary spawn periods, respectively. Although associated with spawning activity, it also appears that seasonal changes in growth rate are attributed to their response to desiccative conditions and temperature extremes3.
In a controlled study by Mau and Jha37, feeding, growth performance and mortality of C. sandwicensis were negatively affected by mean air temperatures >28.5 °C. These captive limpets also exhibited similar seasonal trends observed in CW2—growth rates were lowest in Fall and highest in Winter—and further indicates that growth follows changes in climate.
All limpets in the current study exhibited determinate growth (Fig. 6), where predictable changes in daily growth rate occur during ontogenetic shift from juvenile to adulthood (See Supplementary Table 3). This type of growth, exhibited by yellowfoot limpet, is common in Tropical Pacific gastropods38.
Age-at-maturity is an important metric for understanding population dynamics and assessment of managed stocks. For our isotopically analyzed yellowfoot limpet, age-at-maturity was 8–9 months (~21 mm shell length) which differed from a prior report of 4–7 months (Kay et al., 1981). The discrepancy between current and previous reports most likely arose because the previous authors assumed limpets <5 mm to be 1 month in age.
There was intra-species seasonal variability in growth, where highest mean daily growth rates were observed in May (CW1) and January (CW2), respectively. Frequency of growth, however, was consistently highest in Fall, which precedes the primary spawning window. Based on timing of maximal somatic growth and GSI, we understand that C. sandwicensis is likely bulking for reproduction twice, annually (see Supplementary Equation 1 and Supplementary Fig. 1).
Monthly growth rates changed with frequency of growth days, and consistent growth cessation periods aligned with previously described spawning periods in both Winter and Summer months (longest period of missing growth was ~3 months). The differences in seasonal growth patterns between individuals from the same population likely result from genetic variability (circadian rhythm) and environment (food availability and micro-habitat).
Longevity
Our sclerochronology of temporally aligned shell records provides the first reliable age estimate of C. sandwicenesis. The historical specimen represents the maximal size (68.6 mm SL), and indicates this species to live up to 5 years. The longevity of C. sandwicensis is similar to that reported for related species: C. tramoserica (3yrs), C. radiata (4yrs), C. eucosmia (5yrs), and C. karachiensis (6yrs), which indicates that Cellana is a relatively short-lived clade39,40,41,42.
In theory, the longevity of marine gastropods is selected by environmental and biological pressures to ensure reproductive success43. For C. sandwicensis, longevity may be influenced by wave exposure, average limpet size, population density, substratum type, and human harvesting. Current, ongoing research is focusing on these variables to evaluate their impact to the Hawaiian limpet fishery, and to establish adaptive management strategies.
Methods
Ecology of Yellowfoot limpet
In the Tropical Pacific, sympatric limpets (Cellana melanostoma, Cellana exarata, Cellana sandwicensis, Cellana talcosa) inhabit the Hawaiian rocky intertidal ecosystem, where they graze on crustose coralline algae (CCA) and epibenthic microorganisms. Distribution ranges from the splash zone (upper-intertidal) to subtidal zone, and across the entire Hawaiian Archipelago26. They are dispersed across the majority of seamounts, atolls, and islands, however, not all species are present in every rocky intertidal locality, which reflects species-specific micro-habitat preferences.
The reproduction cycles for each species appears to vary in time and space, and on-going long-term monitoring efforts are in progress to define this critical life-history trait. Previous studies on the yellowfoot limpet C. sandwicensis, reveal that reproduction is highly synchronized from December to March27,29. Gametogenesis also occurs from June to August, however, the level synchronicity and intensity of this second spawn period are inconsistent.
These limpets are gonochoristic and considered to be sequential hermaphrodites44. The sex ratio is near 1:1(M:F) during spawning season, however, we have directly observed populations to maintain disproportionate sex ratios.
Development of this broad-cast spawning limpet has been described from egg to post-larvae, where settlement occurs in less than 4 days post-fertilization29. This short larval duration ensures recruitment to the same localized intertidal environment, and reduces likelihood of hybridization between sympatric species with similar life-histories26.
For wild limpets, growth rates shift through ontogeny—average monthly growth decreasing from 4–5 mm shell length (SL) as juveniles to 2–3 mm SL as adults27. Limpets also exhibit seasonal growth patterns—influenced by temperature and feeding28,37. Currently, growth rates of large individuals (>50 mm SL) and species longevity are absent in the literature.
Regional climate and coastal oceanography
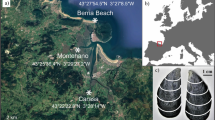
Ka’alawai is located on the south-facing shoreline of Oahu Island, Hawai’i (21°15'20.7“N 157°47'30.8“W). This area, defined as a rocky intertidal zone, is primarily comprised of basalt outcrops, boulders and benches, and supports a diverse community of epibenthic flora and fauna. The area is relatively easy to access by foot, and has been continuously exposed to various anthropogenic factors, which includes development, urban run-off, and subsistence fishing.
The microclimate of the region is characterized by mild, wet winters (January to March) and dry, hot summers (July to September). The mean daily atmospheric temperature range and mean daily sea-surface temperature range are 18.44–31.38 °C and 22.67–30.18 °C, respectively. The annual precipitation is low relative to windward sides of the island, with maximum rainfall of 6.35 cm (data sources: US climate station USC00519397: Waikiki 717.2; PacIOOS Nearshore Sensor 04 (NS04): Waikiki Aquarium). Although freshwater input from precipitation along this coastline is considered to be marginal, the mixing of submarine groundwater discharge generates a unique geochemical profile for surface seawater at Ka’alawai. In particular, the mean surface salinity for this study site has been reported to be 25.4 ‰, which reflects this highly localized land-sea interaction45.
The coastal oceanography of this region is predominantly influenced by wave, wind, and tidal forces. The south-shore region experiences a mixed tidal cycle—having both diurnal and semi-diurnal sinusoidal constituents per lunar day—with a tidal range of 58 cm and 91 cm during neap tide and spring tide, respectively; The trade winds from north-easterly direction (between 22.5°–67.5°) account for ~63% of the year with mean annual intensity around 5 m/s;46 and South swells with wave amplitudes of ~3 m are generated by storms in the Tasmanian Sea during Northern Hemisphere Summer47,48.
Modern and historical specimens
On June 28th of 2018, live Yellowfoot limpet (Cellana sandwicensis) specimens CW1 and CW2 were collected from the rocky intertidal zone at Ka’alawai, Oahu, Hawai’i (Fig. 7). The animals were immediately sacrificed/dissected using scalpel blade, and measured for shell dimensions using a caliper. Limpets were weighed to determine gonadosomatic index, and gonads were preserved for histological examination. Shells were rinsed in an ultrasonic bath and air-dried.
A historical specimen BPBM (identification number 250851-200492) was loaned from the Bernice Pauahi Bishop Museum Malacology Department Collection. This specimen’s geographical and ecological origin is unknown, but was identified as C. sandwicensis by its characteristic shell morphology49. This specimen was selected for its large size to estimate life-expectancy of this limpet species, as well as to evaluate this method for paleoclimatology studies.
Permission was not required to obtain specimens used in this study, and limpets were collected at a size exceeds the legal minimum shell length of 31.8 mm (Hawaii State Law is enforced by Department of Land and Natural Resources). Ethical approval was not required to conduct analysis.
Characterization of shell microstructure
Shell microstructure was identified before isotopic analysis could be attempted. Each shell was cross-sectioned from anterior to posterior direction using a low speed saw (Isomet 1000, Buehler) equipped with a 0.5 mm diamond coated blade. Parallel cuts were made at the apex or maximal growth-axis to obtain two replicate 1.3 mm thick-sections per specimen. The first replicate thick-sections, prepared for micro-sampling, were further cut into <15 mm long pieces and mounted on a single glass round, and the second replicate thick-sections, prepared for sclerochronology, were mounted in its entirety on a large glass slide. Specimens were mounted in using quick-drying epoxy (EPO-TEK 301, Epoxy Technology Inc, Billerica, MA) set in a mold, grinded with F1000 grit SiC powdersecosecondar, and polished with 3 and 1 µm Al2O3 powder on a lapping wheel. Polished sections on glass rounds were then sonicated, rinsed with methanol, and carbon coated to ~250 Å (Cressington Carbon Coater 208carbon, Watford UK). Microstructures of unstained specimens were identified by Scanning Electron Microscopy (SEM; JEOL JSM-5900LV, USA) photomicrograph following MacClintock50.
Raman spectroscopy was used to characterize biogenic carbonate mineralogy by comparing shifts in relative peak position and intensity between calcite and aragonite polymorphs51 (see Supplementary Fig. 4). A silicon wafer standard was used to determine spectral center when grating was 800 grooves/nm. Single spectrum analysis was performed in each microstructure layer using a green laser at 514 nm. A total of six (n = 6) sampling sites were selected haphazardly for a given microstructure layer, which comprised of ten accumulations averaged across 10,000 s. The shell’s exterior surface layer did not return clear spectral peaks, and thus has been excluded from our analysis.
Secondary ion mass spectrometry analysis
Hawaiian limpet Cellana sandwicensis shells CW1, CW2, and BPBM were analyzed for oxygen isotopes. Polished thick-sections were imaged by light and scanning electron microscopy (SEM) to guide sampling by ion microprobe. We sampled in the crossed-foliated, calcite layer (M + 2) to avoid mixing calcite and aragonite layers, and sequential measurements were performed moving from the shell margin toward the apex along the growth axis.
To achieve sub-weekly resolution for an annual δ18O cycle, modern specimens, CW1 and CW2, were analyzed using an average interval of 252 µm and 288 µm between samples, respectively. To achieve sub-annual resolution across multiple δ18O cycles, the historical specimen, BPBM 250851-200492, was analyzed using an average interval of 554 µm. The total number of microprobe sample measurements was 55, 43, and 56 for CW1, CW2, and BPBM, respectively. A single, annual isotope cycle was analyzed for the modern specimens, and four annual isotope cycles were analyzed for the historical specimen.
The carbon-coated thick-sections were placed under vacuum conditions to prevent contamination prior to being measured by CAMECA-IMS-1280 ion microprobe (SIMS; W.M. Keck Research Laboratory, University of Hawai’i) for oxygen isotopes. The primary ion beam Cs+ was set at 2.5 nA for 120 s presputtering. Ions were extracted at ~8 kV. Microprobe rastered across a 15 µm2 area, which accounted for 1-3 lunar daily growth increments. Each measurement included 30 cycles with 10 s integration period. The 16O and 18O were measured in multicollection mode using two Faraday cups with 1010 and 1011 Ω registers, respectively. The b-field was controlled by nuclear magnetic resonance. Mass resolving power was 1958 % min-1, which allows detection of possible interference ions. To correct for instrumental isotope mass fractionation, University of Wisconsin Calcite, UWC-3 (δ18O = 12.49‰ Vienna Standard Mean Ocean Water – VSMOW), was measured consecutively before and after performing microprobe analyses for each specimen (n = 12)52. Corrections were made based on these groups of standard measurements with limited monitoring for instrumental drift. The reproducibility measurements (2σ) of UWC-3 reference material ranged from 0.17 to 0.35‰, which reflected measurement precision and reproducibility of standard measurements for same-day measurements. Measurement errors are reported as 2σ, which reflects both precision (2 standard error) and reproducibility (2 standard deviation) (see Supplementary Data 1 and Supplementary Data 2). Following the microprobe analyses, shell samples were imaged under SEM to expose sample scars for sclerochronology.
Predicted shell δ18O
To examine if Hawaiian limpet shells are in isotopic equilibrium with their environment, we compared measured δ18Ocalcite to predicted δ18Oseawater calculated from seawater-surface temperatures (SST) and surface seawater salinity (SSS). Sea-surface temperatures were obtained from in-situ PacIOOS Nearshore Sensor 04 (NS04): Waikiki Aquarium, Oahu, Hawai’i at 21.26587° N, -157.82275°W (Sea-Bird Electronics model 37SMP, ±0.002 accuracy from -5 to 35 °C). Predicted values were calculated using the equilibrium fractionation equation for calcite and water described by Friedman and O’Neil:53
Where T is temperature in Kelvin and α is the fractionation between calcite and water from the equation:
Where δ18Ocalcite and δ18Oseawater are in VSMOW. Conversion from VPDB to VSMOW scales were performed using the following equation:54
The relationship between mean annual surface seawater δ18O and mean annual surface salinity for the Tropical Pacific region (50-year data set) was used to calculate δ18Oseawater (Legrande & Schmidt 2006):
Mean surface seawater salinity was 25.4 ‰ for Ka’alawai45.
The temperature error for proxy measurements for CW1, CW2, and BPBM were±1.54,±1.53, and±1.60 °C, respectively.
Temporal alignment of shell δ18O
Following SEM and SIMS, we removed the carbon coat with methanol and Kimwipe tissue. The same thick-sections analyzed by microprobe were stained following the previously mentioned procedure, and imaged by light microscopy. The SEM and light photomicrographs were over-layed using Photoshop to accurately expose the spatial-relationship between SIMS-analyzed points and growth lines/increments. For temporal alignment of isotope measurements, we used predicted δ18Ocalcite values that aligned with major lines as anchoring points. Calendar dates (based on a lunar year) were assigned for every isotope measurement between these anchors by counting micro lines (lunar daily growth). We applied previous research on growth and reproduction to resolve alignment discrepancies between predicted δ18Ocalcite and measured δ18Ocalcite.
Climate reconstruction of historical shell
The exact location from which the historical specimen BMBP was collected from is unknown. Climate was reconstructed from the shell isotope record—assuming that δ18Ocalcite is precipitated in equilibrium with δ18Oseawater. Sea surface temperature was calculated from sequentially sampled isotope measurements across ecologically relevant salinity values. Based on the profile with the most biologically relevant temperature thresholds (min and max), we predicted historical sea surface salinity (SSS).
Growth measurements
The polished over-sized, thick-sections were stained with Mutvei’s solution to expose major lines, micro lines, and micro increments by light microscopy55,56,57. Shell thick-sections were placed in a petri dish and submerged in Mutvei’s solution for 45 min held constant at 37–40 °C with constant stirring. These stained thick-sections were imaged using Nikon Eclipse E600 Polarizing light microscope at 100x magnification for performing growth line measurements.
Daily growth was measured along two axes using the standard measuring tool in ImageJ. The first type of daily growth was measured between two micro increments along the growth axis. The second type of daily growth was measured along the horizontal axis (anterior to posterior orientation).
For the latter, we recorded x-coordinates for each point where a micro increment band intersected the M + 3 layer, and subtracted x-coordinates of sequential points to calculate horizontal distance or growth. Back-calculated shell length measurements were used to model age-at-length data (see Supplementary Eq. 2, Supplementary Fig. 2).
We analyzed sub-monthly growth of modern specimens across an annual isotope cycle to understand temporal changes in growth. This period was selected for based on the shell length at which C. sandwicensis enters adulthood, which allows interpretation of adult growth (Kay et al. 1983).
Shell growth model
We used the back-calculated measurements of shell length-at-age as data inputs to estimate parameters of the von Bertalanffy growth function (VBGF), a standard method of describing growth in marine animals58,59. The VBGF (Eq. 5) was fit to shell length (in mm) at age (in months) data for each shell individually (i.e., CW1, CW2, BH) and pooled samples using non-linear parameter estimation:
where L(t) is length (mm) at age t (years), L∞ is the mean asymptotic length (mm), K is the growth coefficient, and t0 is the theoretical age at length zero. Growth curves were fit by constraining \({L}_{0}\,\)to a common shell length of settlement in order to increase the accuracy of VBGF parameter estimates60. The length at post-larval settlement (i.e.,\(\,{L}_{0}\)) of Cellana sandwicensis was obtained from existing literature as 0.254 mm29 and equated with \({t}_{0}\) = -0.09026. To determine if measurements from all shells could be pooled for a single growth model, we used likelihood ratio tests to test for pairwise differences in \({L}_{{{{{{\rm{\infty }}}}}}}\) and K between shells by generating a χ2 statistic for each set of comparisons (sensu;61,62 Haddon, 2011). Growth data for the pairwise comparisons were truncated to a shell length range of 0 – 45 mm that represented the range of data overlap for all three shells to minimize bias from the larger maximum size of shell BPBM61. We used R v3.3.1 (R Core Team, Vienna, Austria), and Excel v2013 (Microsoft Corporation, Redmond, WA, USA) to perform the growth model statistical analyses.
Statistical analysis
Unless stated otherwise, all statistical analyses were accomplished in SAS (SAS v9.2, SAS Institute Inc., Cary, NC, USA). Pearson’s correlation coefficients were computed using Proc Corr to determine linear relationships between SSTcalculated and SSTmeasured. We also used correlation analysis to describe monthly growth by changes in growth frequency and DGSL, respectively. To analyze daily growth as a function of time, we performed univariate ANOVA with repeated measures using Mixed Proc. Pair-wise comparisons of unequal group sizes was performed using Tukeys-Kramer post-hoc analysis. Significant differences were determined using an alpha of 0.05 for all statistical procedures.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data can be found in a publicly accessible repository. The raw and processed temperature data for this study is available at https://doi.org/10.6084/m9.figshare.15050274. The raw and processed growth increment data for modern and historical specimens are available at https://doi.org/10.6084/m9.figshare.15050256. The Bertalanffy growth model for Hawaiian limpet Cellana sandwicensis is available at https://doi.org/10.6084/m9.figshare.15050238. The raw and processed oxygen SIMS-derived oxygen isotope data for modern and historical specimens are available at https://doi.org/10.6084/m9.figshare.15050235.
References
Helmuth, B., Mieszkowska, N., Moore, P. & Hawkins, S. J. Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 37, 373–404 (2006).
Sanford, E., Sones, J. L., García-Reyes, M., Goddard, J. H. & Largier, J. L. Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Sci. Rep. 9, 4216 (2019).
Fenger T., Surge D., Schöne B., & Milner N. Sclerochronology and geochemical variation in limpet shells (Patella vulgata): a new archive to reconstruct coastal sea surface temperature. Geochem. Geophys. Geosystems, 8 (2007).
Hausmann, N. et al. Extensive elemental mapping unlocks Mg/Ca ratios as climate proxy in seasonal records of Mediterranean limpets. Sci. Rep. 9, 3698 (2019).
Nicastro, A. et al. High-resolution records of growth temperature and life history of two Nacella limpet species, Tierra del Fuego, Argentina. Palaeogeography Palaeoclimatol. Palaeoecol. 540, 109526 (2020).
Prendergast, A. L. et al. Oxygen isotopes from Phorcus (Osilinus) turbinatus shells as a proxy for sea surface temperature in the central Mediterranean: a case study from Malta. Chem. Geol. 345, 77–86 (2013).
Prendergast, A. L. & Schöne, B. R. Oxygen isotopes from limpet shells: implications for palaeothermometry and seasonal shellfish foraging studies in the Mediterranean. Palaeogeography Palaeoclimatol. Palaeoecol. 484, 33–47 (2017).
Epstein, S., Buchsbaum, R., Lowenstam, H. A. & Urey, H. C. Revised carbonate-water 480 isotopic temperature scale. Geol. Soc. Am. Bulletin 64, 1315–1326 (1953).
Schöne, B. R., Lega, J., Flessa, K. W., Goodwin, D. H. & Dettman, D. L. Reconstructing daily temperatures from growth rates of the intertidal bivalve mollusk Chione cortezi (northern Gulf of California, Mexico). Palaeogeography Palaeoclimatol. Palaeoecol. 184, 131–146 (2002).
Surge, D., Lohmann, K. C. & Dettman, D. L. Controls on isotopic chemistry of the American oyster, Crassostrea virginica: implications for growth patterns. Palaeogeography Palaeoclimatol. Palaeoecol. 172, 283–296 (2001).
Thompson, R. C., Crowe, T. P. & Hawkins, S. J. Rocky intertidal communities: past environmental changes, present status and predictions for the next 25 years. Environ. Conservation 29, 168–191 (2002).
Tompkins, E. & Adger, W. N. Does adaptive management of natural resources enhance resilience to climate change. Ecol. Society 9, 10 (2004).
Burchell, M., Cannon, A., Hallmann, N., Schwarcz, H. P. & Schöne, B. R. Refining estimates for the season of Shellfish collection on the Pacific Northwest Coast: applying high-resolution stable oxygen Isotope analysis and sclerochronology. Archaeometry 55, 258–276 (2013).
Moss D. K., Ivany L. C., Jones D. S. (2021). Fossil bivalves and the sclerochronological reawakening. Paleobiology, 1–23. https://doi.org/10.1017/pab.2021.16
Wang, T., Surge, D. & Mithen, S. Seasonal temperature variability of the Neoglacial (3300–2500 BP) and Roman Warm Period (2500–1600 BP) reconstructed from oxygen isotope ratios of limpet shells (Patella vulgata), Northwest Scotland. Palaeogeography Palaeoclimatol. Palaeoecol. 317, 104–113 (2012).
Corrège, T. Sea surface temperature and salinity reconstruction from coral geochemical tracers. Palaeogeography Palaeoclimatol. Palaeoecol. 232, 408–428 (2006).
DeLong, K. L., Quinn, T. M., Taylor, F. W., Lin, K. & Shen, C. C. Sea surface temperature variability in the southwest tropical Pacific since AD 1649. Nat. Climate Change 2, 799 (2012).
Dunbar, R. B., Wellington, G. M., Colgan, M. W. & Glynn, P. W. Eastern Pacific sea surface temperature since 1600 AD: The δ18O record of climate variability in Galápagos corals. Paleoceanography Paleoclimatol. 9, 291–315 (1994).
Linsley, B. K., Ren, L., Dunbar, R. B. & Howe, S. S. El Niño Southern Oscillation (ENSO) and decadal-scale climate variability at 10° N in the eastern Pacific from 1893 to 1994: a coral-based reconstruction from Clipperton Atoll. Paleoceanography 15, 322–335 (2000).
Urban, F. E., Cole, J. E. & Overpeck, J. T. Influence of mean climate change on climate variability from a 155-year tropical Pacific coral record. Nature 407, 989 (2000).
Gutiérrez-Zugasti, I. et al. Reprint of Shell oxygen isotope values and sclerochronology of the limpet Patella vulgata Linnaeus 1758 from northern Iberia: implications for the reconstruction of past seawater temperatures. Palaeogeography Palaeoclimatol. Palaeoecol. 484, 48–61 (2017).
Harley, C. D. et al. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (2006).
Schöne, B. R. The curse of physiology—challenges and opportunities in the interpretation of geochemical data from mollusk shells. Geo-Marine Lett. 28, 269–285 (2008).
Smithers, J. & Smit, B. Human adaptation to climatic variability and change. Global Environ. Change 7, 129–146 (1997).
Wefer, G. & Berger, W. H. Isotope paleontology: growth and composition of extant calcareous species. Marine Geol. 100, 207–248 (1991).
Bird, C. E., Holland, B. S., Bowen, B. W. & Toonen, R. J. Contrasting phylogeography in three endemic Hawaiian limpets (Cellana spp.) with similar life histories. Mol. Ecol. 16, 3173–3186 (2007).
Kay E. A., Corpuz G. C., & Magruder W. H. ‘Opihi. Their biology and culture. Report of the Aquaculture Development Program, Department of Land and Natural Resources, State of Hawaii. Honolulu (HI, USA, 1982).
Kay E. A., Bird C. E., Holland B. S., & Smith C. M. Growth rates, reproductive cycles, and population genetics of opihi from the National Parks in the Hawaiian Islands. NPS PICRP Graduate research project final report (2006).
Mau, A., Bingham, J.-P., Soller, F. & Jha, R. Maturation, spawning, and larval development in captive yellowfoot limpets (Cellana sandwicensis). Invertebrate Reprod. Dev. 62, 239–247 (2018).
McCoy, M. D. Hawaiian limpet harvesting in historical perspective: a review of modern and archaeological data on Cellana spp. from the Kalaupapa Peninsula, Moloka ‘i Island1. Pacific Sci. 62, 21–39 (2008).
Morishige, K. et al. Nā Kilo ʻĀina: visions of biocultural restoration through indigenous relationships between people and place. Sustainability 10, 3368 (2018).
Helser, T. E. et al. Evaluation of micromilling/conventional isotope ratio mass spectrometry and secondary ion mass spectrometry of δ18O values in fish otoliths for sclerochronology. Rapid Commun. Mass Spectrom. 32, 1781–1790 (2018).
Wycech, J. B. et al. Comparison of δ18O analyses on individual planktic foraminifer (Orbulina universa) shells by SIMS and gas-source mass spectrometry. Chem. Geol. 483, 119–130 (2018).
Stott, L. et al. Decline of surface temperature and salinity in the western tropical Pacific Ocean in the Holocene epoch. Nature 431, 56 (2004).
Bird, C. E. Aspects of Community Ecology on Wave-Exposed Rocky Hawai’ian Coasts. Doctoral Dissertation, University of Hawaii (2006).
Bird, C. E., Franklin, E. C., Smith, C. M. & Toonen, R. J. Between tide and wave marks: a unifying model of physical zonation on littoral shores. PeerJ 1, e154 (2013).
Mau, A. & Jha, R. Effects of dietary protein to energy ratio on growth performance yellowfoot limpet (Cellana sandwicensis Pease, 1861). Aquac. Rep. 10, 17–22 (2018).
Vermeij, G. J. & Signor, P. W. The geographic, taxonomic and temporal distribution of determinate growth in marine gastropods. Biol. J. Linnean Soc. 47, 233–247 (1992).
Balaparameswara, R. M. Studies on the growth of the limpet Cellana radiata (Born) (Gastropoda: Prosobranchia). J. Molluscan Studies 42, 136–144 (1976).
Emam, W. M. Morphometric studies on the limpet Cellana karachiensis (Mollusca: Gastropoda) from the Gulf of Oman and Arabian Gulf. Indian J. Mar. Sci. 23, 82–85 (1994).
Saad, A. Age, growth and morphometry of the limpet Cellana eucosmia (Mollusca: Gastropoda) from the Gulf of Suez. Indian J. Mar. Sci. 26, 169–172 (1997).
Underwood, A. J. Comparative studies on the biology of Nerita atramentosa Reeve, Bembicium nahum (Lamarck) and Cellana tramoserica (Sowerby) (Gastropoda: Prosobranchia) in S.E. Australia. J. Exp. Mar. Biol. Ecol. 18, 153–172 (1975).
Powell, E. N. & Cummins, H. Are molluscan maximum life spans determined by long-term cycles in benthic communities? Oecologia 67, 177–182 (1985).
Mau A., Fox K., Bingham J.-P. The reported occurrence of hermaphroditism in the yellowfoot limpet (Cellana sandwicensis Pease, 1981). Ann. Aquac. Res. 4, 1045 (2017).
Richardson, C. M., Dulai, H., Popp, B. N., Ruttenberg, K. & Fackrell, J. K. Submarine groundwater discharge drives biogeochemistry in two Hawaiian reefs. Limnol. Oceanography 62, S348–S363 (2017).
Garza J. A., Chu P. S., Norton C. W., & Schroeder T. A. Changes of the prevailing trade winds over the islands of Hawaii and the North Pacific. J. Geophys. Res. Atmos. 117 (2012).
Snodgrass, F. E., Hasselmann, K. F., Miller, G. R., Munk, W. H. & Powers, W. H. Propagation of ocean swell across the Pacific. Philos. Trans. R. Soc. London 259, 431–497 (1966).
Vitousek, S. & Fletcher, C. H. Maximum annually recurring wave heights in Hawai’i. Pacific Sci. 62, 541–554 (2008).
Bird, C. E. Morphological and behavioral evidence for adaptive diversification of sympatric Hawaiian limpets (Cellana spp.). Integr. Comparative Biol. 51, 466–473 (2011).
MacClintock, C. Shell structure of patelloid and bellerophontoid gastropods (Mollusca). Peabody Mus. Nat. History Bull. 22, 140 (1967).
Urmos, J., Sharma, S. K. & Mackenzie, F. T. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Mineral. 76, 641–646 (1991).
Kozdon, R., Ushikubo, T., Kita, N. T., Spicuzza, M. & Valley, J. W. Intratest oxygen isotope variability in the planktonic foraminifer N. pachyderma: real vs. apparent vital effects by ion microprobe. Chem. Geol. 258, 327–337 (2009).
Friedman I., O’Neil J. R. Data of geochemistry: compilation of stable isotope fractionation factors of geochemical interest. In: Data of Geochemistry. U.S.G.S. Professional Paper 440-KK, 1–12 (1977).
Coplen, T. B., Kendall, C. & Hopple, J. Comparison of stable isotope reference samples. Nature 302, 236–238 (1983).
Mutvei, H. On the internal structures of the nacreous tablets in molluscan shells. Scan Electron Microscopy 1979, 457–462 (1979).
Mutvei H., Westermark T., Dunca E., Carell B., Forberg S. Methods for the study of environmental changes using the structural and chemical information in molluscan shells. Bulletin de l’Institut Océanographique (Monaco), 163–186 (1994).
Schöne, B. R., Dunca, E., Fiebig, J. & Pfeiffer, M. Mutvei’s solution: an ideal agent for resolving microgrowth structures of biogenic carbonates. Palaeogeography Palaeoclimatol. Palaeoecol. 228, 149–166 (2005).
Ricker, W. Computation and interpretation of biological statistics of fish populations. Bull. Fisheries Res. Board Canada 191, 1–382 (1975).
Rogers, A. J. & Weisler, M. I. Assessing the efficacy of genus-level data in archaeomalacology: a case study of the Hawaiian Limpet (Cellana spp.), Moloka’i, Hawaiian islands. J. Island Coastal Archaeol. 15, 28–56 (2019).
Kritzer, J. P., Davies, C. R. & Mapstone, B. D. Characterizing fish populations: effects of sample size and population structure on the precision of demographic parameter estimates. Can. J. Fish. Aquat. Sci. 58, 1557–1568 (2001).
Kimura, D. K. Likelihood methods for the von Bertalanffy growth curve. Fish. Bull. 77, 765–776 (1980).
Kirch, P. V. The ecology of marine exploitation in prehistoric Hawaii. Human Ecol. 10, 455–476 (1982).
Acknowledgements
The authors would like to thank Drs. Robert Dunbar and Brian Popp for discussion on stable isotopes, and for technical assistance on microstructure identification and isotope analysis of biogenic carbonate; JoAnn Sinton for lapidary services; the Malacology Department of Bernice Pauahi Bishop Museum for loaning archived historical specimens for research. This work was funded in-part by: the Center for Tropical and Subtropical Aquaculture subaward 2017-292 of USDA/NIFA award 2016-38500-25751 (JPB); the Undergraduate Research Opportunities Program, Office of the Vice Chancellor for Research at the University of Hawaiʻi at Mānoa (JPB/AV); the University of Hawaiʻi at Mānoa Graduate Student Organization grant award 18-02-20 (AM), Hawaiian Malacological Society Student Research Scholarship (AM); and NOAA award NA10NMF4520163 (ECF).
Author information
Authors and Affiliations
Contributions
A.M. conceived the work and wrote the manuscript. A.M, A.V. and P.N. collected live limpets, prepared thin-sections for analysis, and collected isotope data. A.M., G.H. and K.N. processed and analyzed SIMS-derive oxygen isotope data. A.M. and A.V. measured and processed growth increment data. E.F. produced growth model. A.M., E.F. and J-P.B. discussed and revised manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Sze Ling Ho, Clare Davis. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mau, A., Franklin, E.C., Nagashima, K. et al. Near-daily reconstruction of tropical intertidal limpet life-history using secondary-ion mass spectrometry. Commun Earth Environ 2, 171 (2021). https://doi.org/10.1038/s43247-021-00251-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-021-00251-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.