Abstract
The Cretaceous–Paleogene mass extinction event 66 million years ago eradicated three quarters of marine and terrestrial species globally. However, previous studies based on vertebrates suggest that freshwater biota were much less affected. Here we assemble a time series of European freshwater gastropod species occurrences and inferred extinction rates covering the past 200 million years. We find that extinction rates increased by more than one order of magnitude during the Cretaceous–Paleogene mass extinction, which resulted in the extinction of 92.5% of all species. The extinction phase lasted 5.4 million years and was followed by a recovery period of 6.9 million years. However, present extinction rates in European freshwater gastropods are three orders of magnitude higher than even these revised estimates for the Cretaceous–Paleogene mass extinction. Our results indicate that, unless substantial conservation effort is directed to freshwater ecosystems, the present extinction crisis will have a severe impact to freshwater biota for millions of years to come.
Similar content being viewed by others
Introduction
Biodiversity in freshwater ecosystems is disproportionally high: while only covering 1% of the Earth’s surface, they account for about 10% of the global species richness1. These environments and their biota provide invaluable ecosystem services sustaining human health, nutrition and fresh water supply2,3.
Freshwater ecosystems and their biota worldwide are currently experiencing massive deterioration1,4,5,6,7,8,9,10. In many cases, regional species richness and individual abundances are in decline and extinction rates are alarmingly high11,12,13,14. Human impact is widely perceived as the main cause for this decline, either directly through habitat degradation or destruction or indirectly via global warming, eutrophication, pollution, groundwater extraction or invasive species3,11,15,16.
The current biotic crisis is widely considered the onset of a major extinction event, the so-called ‘6th mass extinction’17,18. It resembles in several aspects the 5th mass extinction at the Cretaceous–Paleogene (K–Pg, formerly K–T) boundary 66 million years (Myr) ago. Both biotic crises are in geological timescales catastrophic events—an asteroid impact in the end-Cretaceous versus human impact during the Anthropocene, both paired with a steep rise in CO2 and global temperature19,20,21,22,23,24.
While globally 76% of all species are estimated to have gone extinct during the K–Pg event17, some estimates of diversity loss in freshwater species amount to only 10–22%25. These comparatively low estimates are exclusively based on the vertebrate fossil record. Vertebrates make up around 15% of species diversity in today’s freshwater habitats26, and this low proportion may not warrant general conclusions about the freshwater biota as a whole. The stark discrepancy between the suggested low impact of the 5th mass extinction event and the current, alarmingly high extinction rates and risks for species living in fresh water calls for a reassessment of the K–Pg event beyond freshwater vertebrates.
Freshwater gastropods provide a useful model taxon to assess extinction and diversity recovery. They are among the most diverse groups of animals in freshwater ecosystems today26,27 and have one of the best-preserved fossil records28,29. They inhabit nearly all freshwater habitats worldwide and have evolved different lifestyles and reproductive strategies and occupy different trophic levels28. Moreover, current threats and their response to climate change are well understood and representative of the general threats facing freshwater biodiversity as a whole10,27,29,30,31,32,33.
To evaluate the effects of the 5th mass extinction event on European freshwater biota we compiled and analyzed the fossil record of freshwater gastropods comprising 3122 species to assess the magnitude, duration and dynamics of extinction and of the diversity rebound across the K–Pg boundary. Considering the similarity of that extinction event with the current biodiversity crisis in terms of its catastrophic nature combined with global warming, we use the results from our analyses to outline/predict the pathway of the current crisis for the European biota. For doing so, we use a recently developed approach based on species conservation status obtained from the IUCN Red List34 to infer the magnitude and rate of extinction in Europe’s extant freshwater gastropod fauna12,35. This allows us to evaluate the predicted loss of freshwater gastropod biodiversity in the near future under a Business-as-Usual Scenario22 in comparison to historic extinction events. We use Europe as a model region for our analyses because of its exceptionally rich and well-studied fossil record and thorough conservation assessment of modern species, compared to other regions of the World.
Results
We estimated speciation and extinction times for the fossil record of European freshwater gastropods through a joint inference of speciation, extinction and preservation rates from 24,759 fossil occurrences covering approximately 200 Myr (Supplementary Fig. 1, Supplementary Data 1). The reconstructed species richness trajectory shows a slow evolution throughout the Mesozoic, with only two notable peaks at the Jurassic/Cretaceous boundary and in the Late Cretaceous (Fig. 1a). Diversity drops at the end of the Cretaceous, with on average 92.5% (95% credible interval: 88.0–94.7%) of all species and 9.5% (3.5–15.8%) of all genera going extinct (Fig. 1b). Species richness recovered c. 7–8 Myr later to Late Cretaceous levels and reached a plateau in the Eocene (c. 45–30 Myr ago). Following a decline in the Oligocene, diversity peaked in the Neogene, paralleling major radiations in several long-lived lakes in the middle and late Miocene29,36.
a Inferred species richness from the Jurassic to the Pleistocene based on our diversification analysis. Shown are the mean richness and the 95% highest posterior density interval, as well as a fossil representative of the Late Cretaceous, Pyrgulifera armata (Matheron, 1843) from the Santonian/Campanian of Hungary (photo M. Harzhauser, scale bar = 1 cm; after Bandel & Riedel64). b Survivor ratio of species and genus richness before and after the K–Pg extinction event (gray bar), which is marked by shifts in the extinction rate (gray lines). By the end of the extinction event, 92.5% of all freshwater gastropod species and 9.5% of the genera had gone extinct.
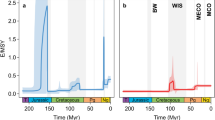
Both speciation and extinction rates are highest at the K–Pg boundary, exceeding the previous levels by more than one order of magnitude (Fig. 2). Earlier speciation rate peaks at the Early–Late Cretaceous transition (100.5 Myr ago) and in the Late Cretaceous (85 Myr ago) are lower and characterized by high uncertainty (wide 95% credible intervals). The extinction rate remains constant throughout most of the Late Cretaceous and Paleogene.
a Speciation rate. b Extinction rate. c Net diversification rate, calculated as speciation minus extinction rate. Shown are the median rates and the 95% highest posterior density quantifying the uncertainty in rates. Here we focus on the Late Cretaceous and the Paleogene; the complete version is shown in Supplementary Fig. 2. E/MSY, events per million species years.
The extinction event at the K–Pg boundary occurs after a period of low extinction and speciation rates in the latest Cretaceous, with a net diversification close to zero (Fig. 2c). The extinction phase involves an interval of 5.4 Myr (66.9–61.4 Myr ago; Fig. 2b). The fact that the first shift in the extinction rate slightly predates the K–Pg boundary is likely a result of the stratigraphic binning of the fossil record being too coarse to precisely date the onset of the extinction event. The extinction event is accompanied by a peak in speciation of similar magnitude (Fig. 2b). While the extinction rate drops to a low level at 61.4 Myr ago (95% credible interval: 61.1–64.3 Myr ago), the speciation rate remains comparatively high for the next 6.9 Myr (3.6–8.7 Myr) until it drops to a similar level as the extinction rate. From 55.1 Myr ago (53.8–56.9 Myr ago) onwards—11.8 Myr after the onset of the event—the net diversification rate is back to Late Cretaceous background level.
Predictions for the current biodiversity crisis based on conservation statuses of 347 extant European freshwater gastropod species assessed by the IUCN Red List34 indicate much higher extinction rates than for the K–Pg event (Supplementary Data 2). Extinction rates range between 1378.9 (1316.7–1441.1), 1787.1 (1733.3–1840.8) and 1973.2 (1923.4–2023.0) E/MSY (events per million species years) over the next 50, 80 and 100 years, respectively (Fig. 3). This is approximately three orders of magnitudes higher than the extinction rate during the K–Pg event (~1.45 E/MSY; Fig. 2b) and reflects the extremely fast pace of the current biodiversity crisis. Further, the predictions indicate that between 72 (20.8%) and 111 species (31.9%) might go extinct during the next 50 to 100 years (Fig. 3). If this trend is projected further into the future, the 75% extinct species threshold defining a mass extinction17 would be reached already by the year 2539 (95% credible interval: 2527–2551). Accordingly, the European freshwater gastropod fauna might suffer from a severe regional extinction event within the millennium.
Predicting the number of extinct species and conservation status change over the next 50, 80 and 100 years based on current species conservation status as derived from the IUCN Red List34 using the recently developed program iucn_sim35. The graph shows the results from the dataset excluding species with little fossilization potential (spring, subterranean, cave and karstic species) to facilitate comparison with the results from the diversification analysis. The results of the analysis based on the complete fauna are provided in the Supplementary Data 2 and Supplementary Fig. 3.
We find a similar extinction rate when using genera instead of species, i.e., 1018.7 (852.0–1199.6) E/MSY over the next 100 years. This rate is unsurprisingly lower than that for the species-level analyses and likely relates to half of the genera in the IUCN dataset containing at least one non-threatened species. Nonetheless, the result still ranges in the same order of magnitude as the species-level analyses showing that our results are robust and do not depend on the systematic level.
Discussion
Based on the rich European gastropod fossil record, we show that freshwater extinction at the K–Pg boundary was severe and has been underestimated so far. Previous studies based on the global vertebrate fossil record reported freshwater species extinction levels of 10–22%25. However, our new estimates based on freshwater gastropods show a species richness decline in Europe of 92.5% and an extinction rate being more than an order of magnitude higher than background level. This magnitude is even higher than the global average of the 5th mass extinction event, which wiped out approximately 76% of all species on Earth17. As such, freshwater gastropods line up with major terrestrial and marine animal and plant groups that experienced strong decline in species richness or disappeared entirely. This concerns, for example, non-avian dinosaurs, ammonites, bivalves, ostracods, planktic foraminifera, calcareous nannofossils, bryozoans and land plants19,37,38,39,40.
Whether the past extinction pattern we found based on the European fossil record represents a global phenomenon remains uncertain. We are currently lacking comprehensive records for most continents (with the exception of North America41) that have sufficient detail to infer diversification rates. The growing availability of quality-controlled fossil data in unified and open repositories is an essential precondition to expand regional studies to a global scale in future research.
The 5th mass extinction event shares several features with the current crisis, making it a potential model system to investigate and predict biotic response to the catastrophic environmental turnover we observe today. During both the latest Cretaceous and the late Cenozoic, a global cooling trend is followed by a steep and rapid temperature increase driven primarily by rising CO2 levels20,21,22,23,42. Both intervals witness concerted biodiversity decline in many taxonomic groups29,43,44 (see also Fig. 1), but peaks in extinction rates coincide with catastrophic events. The current biodiversity crisis is largely the result of human impact6,12,16,45,46,47, and its tempo (decades to millennia) is that of an instantaneous event in terms of the geological time scale. Thus, one may consider the catastrophic event at the K–Pg boundary a suitable parallel for the current, anthropogenically driven crisis.
Comparing our reconstructions of diversification dynamics and extinction magnitude for the 5th mass extinction event with the predicted future trends of the current biodiversity gives deeply worrying prospects. Projected extinction rates of European freshwater gastropods are estimated to be approximately three orders of magnitudes higher than for the 5th mass extinction event (1378.9 to 1973.2 versus 1.45 E/MSY), indicating a much faster pace of extinction than at the K–Pg boundary. These values predicted for the near future are in a similar range as previous estimates for the current biodiversity crisis12,17,46. Also, they greatly exceed other regional extinction events in the European fossil record. For example, the extinction rate of freshwater gastropods has risen already over the last few million years and reached an intermediate plateau during the Quaternary (~0.9 E/MSY; Supplementary Figure 2). While this rate is clearly above the natural background rate of extinction, probably reflecting the massive impact of intensifying glacial cycles on freshwater biota during the Quaternary33,48,49, the present peak is three to four orders of magnitude higher. According to our model, 75% of all European freshwater gastropod species might be lost before the end of the millennium, if the current trends are not reversed. If human impact—either directly via habitat degradation or destruction, eutrophication or pollution or indirectly through climate change—continues to increase as predicted11,16, we will steer into a regional extinction crisis of unprecedented magnitude even earlier.
The aftermath of the K–Pg event in Europe also implies an extended recovery period for the current crisis, which could last millions of years. Even after the immediate cause of extinction at the K–Pg boundary ceased (i.e., the asteroid impact and extreme weather conditions in the successive months/years), the extinction rate remained high for 5.4 Myr and the subsequent recovery period involved another 6.9 Myr. Acknowledging uncertainties in data and model, it took approximately 12 Myr until net diversification returned to the Late Cretaceous level (Fig. 2). Gastropod species richness took approximately 7–8 Myr to recover (Fig. 1). Translating this to the current biodiversity crisis suggests that even if anthropogenic impact on the world’s biota ceases immediately, the already triggered phase of extinction might still involve several million years and so will faunal recovery50,51. Given the extremely fast pace at which global change happens today and the high rates of extinction predicted for the next decades, both of which outpace the rates for the 5th mass extinction, Europe’s freshwater biota may face an even more dramatic collapse in the near future than after the asteroid impact 66 Myr ago.
The ongoing extinction crisis has consequences on many levels. For individual ecosystems, the loss of species comes with an inevitable change in community composition. Species perform specific functions in ecosystems and—depending on their role and interactions with other species—their extinction or local extirpation may have immediate causes on the ecosystem stability or functioning45,52,53,54. Radical changes in ecosystem functioning, in turn, may have severe implications on ecosystem services for humanity, such as food provision, disease resistance or economic benefits11,45,50. Thus, if we continue to lose species at the fast pace our analysis suggests, we will continue to impair ecosystem services to our own disadvantage.
In summary, our study clearly evidences the vulnerability of freshwater biota to past and current global change and implies a long aftermath in case we cannot mitigate the ongoing extinction crisis. We show that rates of extinction are by one (66 Myr ago) or four (today) orders of magnitudes higher than background level. 92.5% of all freshwater gastropod species went extinct during the K–Pg event in Europe, which entailed an extinction phase lasting 5.4 Myr and a subsequent recovery period lasting further 6.9 Myr. The current biodiversity crisis appears even more drastic, with species being lost at a much faster pace. Our analyses suggest that 75% of all European species may be lost within centuries. Our findings provide yet additional evidence that immediate and effective action is needed to protect biodiversity. While our study is restricted to the European continent, we see similar developments in freshwater biota world-wide1,4,5,6,7,8,9,10, indicating that the regional extinction event we predict for European freshwater biota is not an isolated case. Future studies need to compare fossil and modern data from other continents—as far as the fossil record allows—to make general predictions for biota across the globe.
Methods
Fossil occurrence data
Given the inequity in species richness and distribution of the freshwater gastropod fossil record across the planet, we focus on Europe as target region. The European fossil record is well studied as evidenced by a rich and long research history and provides a high number of species and good geographic coverage (Supplementary Fig. 1, Supplementary Data 1).
Locality-based freshwater gastropod occurrence data for the Jurassic–Pleistocene (201.3–0.0117 Myr ago) of Europe were assembled from the literature and selected collection sources (Supplementary Fig. 1, Supplementary Data 1). Most data for the Neogene and Quaternary derive from a previous study29 yet incorporating recent taxonomic revisions and additions. The geographic delimitation of Europe follows that study and includes Anatolia and the Caucasus region; in the east, it is bounded by the Ural Mountains. Data from western Turkmenistan and Kazakhstan were included if associated with the biogeographic realms of the Caspian Sea and Paratethys Sea, respectively. Despite major paleogeographic changes affecting the connectivity of Europe and adjacent continents over the past 200 Myr55, faunas from coeval strata on other continents show very different community compositions41,56,57. These differences suggest the presence of clear biogeographic boundaries allowing a delimitation of the European freshwater gastropod fauna through geological time.
We focused on species as the basic entity for estimating diversification rates. Diversity reconstructions in deep time have been typically performed on genus- or family-level data44,58, but especially for stratigraphically old faunas, such as the early relatives of modern clades back in the Jurassic and Cretaceous, genus or even family classifications are often doubtful. Species are typically binned into common genera, which may greatly inflate actual genus ranges. Similarly, many Mesozoic species lack sufficient diagnostic features that allow clear assignment to a specific family. It has also been shown that the use of genus-level data can severely bias the outcomes of evolutionary studies59, many of which currently focus on the species-level data35,50,51,54.
We applied a careful taxonomic screening process to ascertain a sound data basis for the analyses. Uncertain records (“cf.”), nomina nuda, nomina dubia and taxa inquirenda were excluded. Taxa with temporary names were included; although their names might not be accepted at present (e.g., being junior homonyms pending revision), they still represent valid species. We applied the latest taxonomic revisions to account for changes to species lists, synonymizations, taxon rank and systematic classification. To assure a sound age classification of the fossil occurrences, we used most up-to-date stratigraphic information for the fossil-bearing strata and accounted for revisions in regional stratigraphic schemes and lithostratigraphic and biostratigraphic correlations. Ecologically, we included obligate freshwater as well as oligohaline taxa; mesohaline (e.g., mangrove or estuary) species were excluded.
In the end, the data were critically reviewed by the authors, some of which are experts in non-marine gastropods taxonomy and systematics as well as stratigraphy. Selected records or faunas that appeared highly doubtful due to severe taxonomic or stratigraphic discrepancies were excluded to avoid bias from misidentifications. The final dataset includes 24,759 occurrences of 3,122 species in 5,564 localities (Supplementary Fig. 1, Supplementary Data 1).
Despite the huge amount of data available, extant European faunas were not considered in the present study for a number of reasons: (1) Preliminary analyses have shown that the discrepancy in the number of records available for fossil and recent faunas in relation to their stratigraphic ranges complicates the inference of the diversification rate; analyses regularly fail to converge even when using a high number of generations. (2) Taxonomic and species concepts tend to vary between neontologists and paleontologists, owed to the different preservation and species traits of the material available to them. Including both sources of data will inevitably result in taxonomic biases. (3) The selective preservation towards lacustrine or lowland faunas in the fossil record28 complicates comparison with the recent fauna characterized by numerous spring and subterranean species. Accordingly, we limited the dataset to pre-Holocene records.
Inferring the diversification rate
In order to account for age uncertainties, we generated 200 randomized data sets by resampling the age of each occurrence uniformly within the respective temporal range, following the procedure described by Silvestro et al.60. Analyses were performed using the open-source program PyRate60 (https://github.com/dsilvestro/PyRate). Fossil occurrences are modeled as the joint result of preservation potential (i.e., the probability of sampling through time and including its heterogeneity across species), speciation and extinction. Sampling is modeled as a time-variable Poisson process according to stratigraphic epochs, with the exception of constraining a time-invariable sampling rate for the Early and Middle Jurassic because of the paucity of records for these time intervals. We inferred species diversification using a Reversible Jump Markov Chain Monte Carlo (RJ-MCMC) algorithm to infer time-varying speciation and extinction rates, where rates are allowed to shift over time60.
Analyses were divided into two steps. Firstly, to obtain posterior estimates on speciation and extinction times, we ran the analyses with 200,000,000 MCMC iterations at a sampling frequency of 20,000, discarding the first 20% as burn-in. From each resulting MCMC log-file, we extracted three posterior samples of speciation and extinction times. The resulting 600 replicates of speciation and extinction times were used in a second round of analyses to infer the number of rate shifts. We checked the effective sampling size (ESS) with the ‘coda’ package 0.19-361 for the statistical programming environment R 3.6.362 and base all our observations and interpretations on the 100 log-files with highest ESSs (i.e., ESS for birth–death likelihood of > 100 and ESSs for posterior, prior, the number of sampled rate shifts and hyperprior of > 90). We calculated the extinction magnitude across the K–Pg boundary as the percentage of taxa that originated before the event but became extinct during the event (which is defined by two shifts in the extinction rate; see Fig. 2c).
Predicting future extinction magnitude and rates
We used the recently developed program iucn_sim35 to simulate future extinctions based on conservation status information according to the IUCN Red List34. We queried the IUCN Red List for freshwater gastropods occurring in European countries (including Caucasus countries as well as Turkey, to cover the same geographic scope as the fossil dataset). Taxonomically, we included the families listed by the European Red List of non-marine mollusks30, excluding the semiaquatic families Assimineidae and Ellobiidae. Only extant resident species were included; extinct, possibly extinct and introduced species were omitted. For the final dataset, we excluded species occurring exclusively in caves, springs and karstic or subterranean environments. Species living in these habitats have little fossilization potential28 and including them would impede a reliable comparison with the estimates from the fossil dataset. These filters yielded 347 species with valid conservation status. Nonetheless, to allow comparison with other datasets and faunas, we carried out the same set of analyses also on the dataset including the entire fauna (881 species); the results are provided in the Supplementary Data 2. The R package ‘rredlist’ 0.6.063 was used to collect the status history and most recent conservation status for each species.
The dataset was analyzed using the recently developed program iucn_sim35, which is freely available at https://github.com/tobiashofmann88/iucn_extinction_simulator. The analysis models future changes in conservation status for each species based on estimates of status transition rates, allowing to predict the number of species that will become extinct over a defined time interval. For species marked as ‘Data Deficient’ (DD), a new valid status is drawn based on randomly sampling 100 transition rates for all transitions from DD to any of the statuses LC, NT, VU, EN and CR35. Here we applied the model for three time intervals: 50 years, 80 years (corresponding to year 2100) and 100 years (as in Andermann et al.35). Based on the model, we calculated the mean duration until the extant fauna reaches the 75% extinction level, which marks a mass extinction17. Additionally, we employed an MCMC algorithm as implemented in iucn_sim using 100,000 generations (discarding the first 5000 as burn-in) and 50,000 simulations to infer rates of extinction based on the three time intervals using the ‘EX mode 1’ algorithm35.
Predictions become more accurate when accounting for conservation status history and information on species generation lengths35. Unfortunately, we lack both; there are hardly any changes recorded by the IUCN Red List34 as concerns the conservation statuses of European freshwater gastropod species since the first assessments in the 1990s and there is insufficient knowledge about generation lengths of the 347 species. Nonetheless, an analysis excluding the available conservation status history showed that this has a limited effect on the outcome (115.0 vs. 110.8 extinctions for the 100-year scenario; see Supplementary Data 2).
To test for the robustness of the approach with respect to the taxonomic level, we inferred extinction rates for extant genera for the 100-year scenario. Since genera do not have IUCN conservation statuses, we inferred the extinction rate on genus-level by obtaining an extinction date for each and every species and then calculating the inverse of the mean extinction date of the genera’s last survivors. For each species, we drew a random extinction date from an exponential distribution, with a shape defined as the inverse of the extinction rate of the respective species (which itself is a random draw from the uncertainty distribution provided by our original species-level iucn_sim analysis). We repeated the whole procedure of drawing extinction dates to calculate the genus-level extinction rate based on last survivors 1000 times to obtain an average and 95% credible interval. This accounts for the inferred uncertainty of species-specific extinction rates from our species-level analysis and the randomness of sampled extinction dates.
Data availability
The data underlying this study are available at https://doi.org/10.22029/jlupub-9 and in Supplementary Data 1–2.
References
Darwall, W. et al. The alliance for freshwater life: a global call to unite efforts for freshwater biodiversity science and conservation. Aquat. Conserv. 28, 1015–1022 (2018).
Green, P. A. et al. Freshwater ecosystem services supporting humans: pivoting from water crisis to water solutions. Global Environ. Chang. 34, 108–118 (2015).
EEA (European Environment Agency). The European environment — state and outlook 2020. Knowledge for transition to a sustainable Europe (Publications Office of the European Union, Luxembourg, 2019).
Dudgeon, D. et al. Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182 (2006).
Régnier, C., Fontaine, B. & Bouchet, P. Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions. Conserv. Biol. 23, 1214–1221 (2009).
Vörösmarty, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010).
Burkhead, N. M. Extinction rates in North American freshwater fishes, 1900–2010. BioScience 62, 798–808 (2012).
Poff, N. L., Olden, J. D. & Strayer, D. L. Climate change and freshwater fauna extinction risk. 309–336. In: Hannah, L. (ed.) Saving a million species (Island Press/Center for Resource Economics, Washington, 2012).
De Grave, S. et al. Dead shrimp blues: a global assessment of extinction risk in freshwater shrimps (Crustacea: Decapoda: Caridea). PLoS ONE 10, e0120198 (2015).
Böhm, M. et al. The conservation status of the world’s freshwater molluscs. Hydrobiologia (2020) https://doi.org/10.1007/s10750-020-04385-w.
Albert, J. S. et al. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50, 85–94 (2021).
Andermann, T., Faurby, S., Turvey, S. T., Antonelli, A. & Silvestro, D. The past and future human impact on mammalian diversity. Sci. Adv. 6, eabb2313 (2020).
Dudgeon, D. Freshwater biodiversity: status, threats and conservation (Cambridge University Press, Cambridge, 2020).
WWF (World Wildlife Fund). Living Planet Report – 2020: Bending the curve of biodiversity loss (WWF, Gland, 2020).
Döll, P. & Zhang, J. Impact of climate change on freshwater ecosystems: a global-scale analysis of ecologically relevant river flow alterations. Hydrol. Earth Syst. Sci 14, 783–799 (2010).
Janse, J. H. et al. GLOBIO-Aquatic, a global model of human impact on the biodiversity of inland aquatic ecosystems. Environ. Sci. Policy 48, 99–114 (2015).
Barnosky, A. D. et al. Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011).
Ceballos, G. et al. Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015).
Schulte, P. et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 327, 1214–1218 (2010).
Wang, J.-G., Wu, F.-Y., Tan, X.-C. & Liu, C.-Z. Magmatic evolution of the Western Myanmar Arc documented by U-Pb and Hf isotopes in detrital zircon. Tectonophysics 612–613, 97–105 (2014).
Mills, B. J. W. et al. Modelling the long-term carbon cycle, atmospheric CO2, and Earth surface temperature from late Neoproterozoic to present day. Gondwana Res. 67, 172–186 (2019).
Shukla, P. R. et al. (eds) Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems (IPCC, Geneva, 2019).
Sprain, C. J. et al. The eruptive tempo of Deccan volcanism in relation to the Cretaceous-Paleogene boundary. Science 363, 866–870 (2019).
Hull, P. M. et al. On impact and volcanism across the Cretaceous-Paleogene boundary. Science 367, 266–272 (2020).
Robertson, D. S., Lewis, W. M., Sheehan, P. M. & Toon, O. B. K-Pg extinction patterns in marine and freshwater environments: the impact winter model. J. Geophys. Res. Biogeosci. 118, 1006–1014 (2013).
Balian, E. V., Segers, H., Lévêque, C. & Martens, K. The freshwater animal diversity assessment: an overview of the results. Hydrobiologia 595, 627–637 (2008).
Darwall, W., Seddon, M., Clausnitzer, V. & Cumberlidge, N. Freshwater invertebrate life. 26–32. In: Collen, B., Böhm, M., Kemp, R. & Baillie, J. E. M. (eds). Spineless: status and trends of the world’s invertebrates (Zoological Society of London, London, 2012).
Strong, E. E., Gargominy, O., Ponder, W. F. & Bouchet, P. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595, 149–166 (2008).
Neubauer, T. A., Harzhauser, M., Georgopoulou, E., Kroh, A. & Mandic, O. Tectonics, climate, and the rise and demise of continental aquatic species richness hotspots. Proc. Natl. Acad. Sci. USA 112, 11478–11483 (2015).
Cuttelod, A., Seddon, M. & Neubert, E. European red list of non-marine molluscs (Publications Office of the European Union, Luxembourg, 2011).
Cordellier, M., Pfenninger, A., Streit, B. & Pfenninger, M. Assessing the effects of climate change on the distribution of pulmonate freshwater snail biodiversity. Mar. Biol. 159, 2519–2531 (2012).
Markovic, D. et al. Europe’s freshwater biodiversity under climate change: distribution shifts and conservation needs. Divers. Distrib. 20, 1097–1107 (2014).
Georgopoulou, E., Neubauer, T. A., Harzhauser, M., Kroh, A. & Mandic, O. Distribution patterns of European lacustrine gastropods: a result of environmental factors and deglaciation history. Hydrobiologia 775, 69–82 (2016).
IUCN (International Union for Conservation of Nature). The IUCN red list of threatened species. Version 2020-1. https://www.iucnredlist.org (2020).
Andermann, T., Faurby, S., Cooke, R., Silvestro, D. & Antonelli, A. iucn_sim: a new program to simulate future extinctions based on IUCN threat status. Ecography 44, 162–176 (2021).
Neubauer, T. A., Harzhauser, M., Kroh, A., Georgopoulou, E. & Mandic, O. A gastropod-based biogeographic scheme for the European Neogene freshwater systems. Earth-Sci. Rev. 143, 98–116 (2015).
Sheehan, P. M., Coorough, P. J. & Fastovsky, D. E. Biotic selectivity during the K/T and Late Ordovician extinction events. Geol. Soc. Spec. Pap. 307, 477–489 (1996).
MacLeod, N. et al. The Cretaceous-Tertiary biotic transition. J. Geol. Soc. 154, 265–292 (1997).
Vajda, V. & Bercovici, A. The global vegetation pattern across the Cretaceous–Paleogene mass extinction interval: a template for other extinction events. Global Planet. Change 122, 29–49 (2014).
Silvestro, D., Cascales-Miñana, B., Bacon, C. D. & Antonelli, A. Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436 (2015).
Henderson, J. Fossil non-marine Mollusca of North America. Geol. Soc. Spec. Pap. 3, 1–313 (1935).
Steffen, W. et al. Trajectories of the Earth System in the Anthropocene. Proc. Natl. Acad. Sci. USA 115, 8252–8259 (2018).
Bown, P. R., Lees, J. A. & Young, J. R. Calcareous nannoplankton evolution and diversity through time. 481–508. In: Thierstein, H. R. & Young, J. R. (eds). Coccolithophores (Springer, Berlin, 2004).
Alroy, J. et al. Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100 (2008).
Naeem, S., Duffy, J. E. & Zavaleta, E. The functions of biological diversity in an age of extinction. Science 336, 1401–1406 (2012).
Pimm, S. L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014).
Cowie, R. H., Régnier, C., Fontaine, B. & Bouchet, P. Measuring the sixth extinction: what do mollusks tell us? Nautilus 131, 3–41 (2017).
Georgopoulou, E. et al. Beginning of a new age: How did freshwater gastropods respond to the Quaternary climate change in Europe? Quat. Sci. Rev. 149, 269–278 (2016).
Csapó, H. et al. Successful post-glacial colonization of Europe by single lineage of freshwater amphipod from its Pannonian Plio-Pleistocene diversification hotspot. Sci. Rep. 10, 18695 (2020).
Davis, M., Faurby, S. & Svenning, J.-C. Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc. Natl. Acad. Sci. USA 115, 11262–11267 (2018).
Lowery, C. M. & Fraass, A. J. Morphospace expansion paces taxonomic diversification after end Cretaceous mass extinction. Nat. Ecol. Evol. 3, 900–904 (2019).
Cardinale, B. J., Palmer, M. A. & Collins, S. L. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415, 426–429 (2002).
Thompson, P. L., Rayfield, B. & Gonzalez, A. Loss of habitat and connectivity erodes species diversity, ecosystem functioning, and stability in metacommunity networks. Ecography 40, 98–108 (2017).
Pimiento, C. et al. Selective extinction against redundant species buffers functional diversity. Proc. R. Soc. B 287, 20201162 (2020).
Cao, W. et al. Improving global paleogeography since the late Paleozoic using paleobiology. Biogeosciences 14, 5425–5439 (2017).
Martinson, G. G. Mezozoiskie i Kainozoiskie Molliuski kontinentalnykh otlozhenii Sibirskoi Platformy Zabaikalia i Mongolii. Trudy Baikal’skoy Limnologicheskoy Stantzii Akademii Nauk SSSR 19, 1–332 (1961).
Pan, H. Mesozoic and Cenozoic fossil Gastropoda from Yunnan. 83-152. In: Nanjing Institute of Geology and Palaeontology (Ed.). Mesozoic Fossils from Yunnan. 2 (Science Press, Beijing, 1977).
Payne, J. L., Bush, A. M., Heim, N. A., Knope, M. L. & McCauly, D. J. Ecological selectivity of the emerging mass extinction in the oceans. Science 353, 1284–1286 (2016).
Hendricks, J. R., Saupe, E. E., Myers, C. E., Hermsen, E. J. & Allmon, W. D. The generification of the fossil record. Paleobiology 40, 511–528 (2014).
Silvestro, D., Salamin, N., Antonelli, A. & Meyer, X. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. Paleobiology 45, 546–570 (2019).
Plummer, M. et al. coda: Output analysis and diagnostics for MCMC. R package version 0.19-3. https://cran.r-project.org/web/packages/coda/index.html (2019).
R Core Team. R: A language and environment for statistical computing. Version 3.6.3. R Foundation for Statistical Computing, Vienna. http://www.R-project.org (2020).
Chamberlain, S. rredlist: ‘IUCN’ red list client. R package version 0.6.0. http://CRAN.R-project.org/package=rredlist (2020)
Bandel, K. & Riedel, F. The late Cretaceous gastropod fauna from Ajka (Bakony Mountains, Hungary): a revision. Ann. Naturhist. Mus. Wien Ser. A 96, 1–65 (1994).
Acknowledgements
Computational resources were provided by the BMBF-funded de.NBI Cloud within the German Network for Bioinformatics Infrastructure (de.NBI). We are grateful to Burkhard Linke for assistance during the cloud setup; to Tobias Andermann for assistance with the predictions based on IUCN data; to Wolfgang Brunnbauer, Sebastian Calzada, Daniela Esu, Joachim Gründel, Sonja Herzog-Gutsch, Olaf Höltke, Arie W. Janssen, Ronald Janssen, Eike Neubert, Jean-Michel Pacaud, Sophie Passot, Simon Schneider and Maxim Vinarski for providing literature. The constructive comments of three anonymous reviewers greatly improved an earlier draft of the paper. T.A.N. was supported by a Just’us Fellowship, an Alexander-von-Humboldt Fellowship and a DFG grant (NE 2268/2-1). D.S. and T.H. received funding from the Swiss National Science Foundation (PCEFP3_187012; FN-1749). D.S. also acknowledges funding from the Swedish Research Council (VR: 2019-04739).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
T.A.N. and T.W. designed the study. T.A.N., D.K., M.H. and F.P.W. collected and reviewed data on species occurrences, taxonomy and stratigraphy. T.A.N., T.H., J.S. and D.S. performed the analyses. T.A.N. led the writing of the manuscript; all authors contributed to the writing and the interpretation of the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary handling editor: Joe Aslin
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neubauer, T.A., Hauffe, T., Silvestro, D. et al. Current extinction rate in European freshwater gastropods greatly exceeds that of the late Cretaceous mass extinction. Commun Earth Environ 2, 97 (2021). https://doi.org/10.1038/s43247-021-00167-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-021-00167-x
This article is cited by
-
The scope and extent of literature that maps threats to species globally: a systematic map
Environmental Evidence (2022)
-
Onset of Late Cretaceous diversification in Europe’s freshwater gastropod fauna links to global climatic and biotic events
Scientific Reports (2022)
-
Short-term paleogeographic reorganizations and climate events shaped diversification of North American freshwater gastropods over deep time
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.