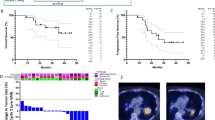
Abstract
Over 500 clinical trials are investigating combination radiotherapy and immune checkpoint blockade (ICB) as cancer treatments; however, the majority of trials have found no positive interaction. Here we perform a comprehensive molecular analysis of a randomized phase I clinical trial of patients with non-small cell lung cancer (NSCLC) treated with concurrent or sequential ablative radiotherapy and ICB. We show that concurrent treatment is superior to sequential treatment in augmenting local and distant tumor responses and in improving overall survival in a subset of patients with immunologically cold, highly aneuploid tumors, but not in those with less aneuploid tumors. In addition, radiotherapy alone decreases intratumoral cytotoxic T cell and adaptive immune signatures, whereas radiotherapy and ICB upregulates key immune pathways. Our findings challenge the prevailing paradigm that local ablative radiotherapy beneficially stimulates the immune response. We propose the use of tumor aneuploidy as a biomarker and therapeutic target in personalizing treatment approaches for patients with NSCLC treated with radiotherapy and ICB.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
DNA and RNA sequencing data that support the findings of this study have been deposited in the European Genome-Phenome Archive (study ID: EGAS00001006212). The human lung adenocarcinoma genomic and clinical data were derived from the TCGA Research Network (http://cancergenome.nih.gov). Previously published NGS data that were reanalyzed here are available in the supplementary material of24 (UC dataset) and at the following cBioPortal study: https://www.cbioportal.org/study/summary?id=tmb_mskcc_2018 (MSKCC dataset). Source data have been provided in Supplementary Tables 1–16. All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
Code for all relevant genomic analyses is available at the GitHub repository for this manuscript (https://github.com/lfspurr/COSINR-Analysis).
References
Herbst, R. S., Morgensztern, D. & Boshoff, C. The biology and management of non-small cell lung cancer. Nature 553, 446–454 (2018).
Gandhi, S. J. et al. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 368, 185–190 (2015).
Shabason, J. E. & Minn, A. J. Radiation and immune checkpoint blockade: from bench to clinic. Semin. Radiat. Oncol. 27, 289–298 (2017).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Weichselbaum, R. R., Liang, H., Deng, L. & Fu, Y.-X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 14, 365–379 (2017).
Chen, H.-Y. et al. Inhibiting the CD8+ T cell infiltration in the tumor microenvironment after radiotherapy is an important mechanism of radioresistance. Sci. Rep. 8, 11934 (2018).
Arina, A., Gutiontov, S. I. & Weichselbaum, R. R. Radiotherapy and immunotherapy for cancer: from ‘systemic’ to ‘multisite’. Clin. Cancer Res. 26, 2777 (2020).
Antonia, S. J. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384, 1191–1203 (2021).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Strickler, J. H., Hanks, B. A. & Khasraw, M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin. Cancer Res. 27, 1236 (2021).
Schumacher Ton, N. & Schreiber Robert, D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Bestvina, C. M. et al. A phase 1 trial of concurrent or sequential ipilimumab, nivolumab, and stereotactic body radiotherapy in patients with stage IV NSCLC study. J. Thorac. Oncol. 17, 130–140 (2022).
Paz-Ares, L. G. et al. First-line nivolumab plus ipilimumab in advanced nsclc: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J. Thorac. Oncol. 17, 289–308 (2022).
Aran, D., Hu, Z. & Butte, A. J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 18, 220 (2017).
Bolotin, D. A. et al. MiXCR: software for comprehensive adaptive immunity profiling. Nat. Methods 12, 380–381 (2015).
Reuben, A. et al. TCR repertoire intratumor heterogeneity in localized lung adenocarcinomas: an association with predicted neoantigen heterogeneity and postsurgical recurrence. Cancer Discov. 7, 1088 (2017).
Joshi, K. et al. Spatial heterogeneity of the T cell receptor repertoire reflects the mutational landscape in lung cancer. Nat. Med. 25, 1549–1559 (2019).
Lamberti, G. et al. Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Ann. Oncol. 31, 807–814 (2020).
Davoli, T., Uno, H., Wooten, E. C. & Elledge, S. J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 355, eaaf8399 (2017).
Taylor, A. M. et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 33, 676–689.e3 (2018).
Gutiontov, S. I. et al. CDKN2A loss-of-function predicts immunotherapy resistance in non-small cell lung cancer. Sci. Rep. 11, 20059 (2021).
Hoadley, K. A. et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 173, 291–304.e6 (2018).
Formenti, S. C. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 24, 1845–1851 (2018).
Golden, E. B. & Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 25, 11–17 (2015).
Jia, Q. et al. Mutational burden and chromosomal aneuploidy synergistically predict survival from radiotherapy in non-small cell lung cancer. Commun. Biol. 4, 131 (2021).
Spurr, L. F. et al. Quantification of aneuploidy in targeted sequencing data using ASCETS. Bioinformatics 37, 2461–2463 (2021).
Wei, J. et al. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci. Immunol. 6, eabg0117 (2021).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Schneider, V. A. et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 27, 849–864 (2017).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The sequence alignment/map format and saMtools. Bioinformatics 25, 2078–2079 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Ellrott, K. et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 6, 271–281.e7 (2018).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Costello, M. et al. Discovery and characterization of artifactual mutations in deep coverage targeted capture sequencing data due to oxidative DNA damage during sample preparation. Nucleic Acids Res. 41, e67 (2013).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. https://doi.org/10.1200/PO.17.00011 (2017).
McGranahan, N. et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci. Transl. Med. 7, 283ra54 (2015).
Blokzijl, F., Janssen, R., van Boxtel, R. & Cuppen, E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Genome Med. 10, 33 (2018).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Zhu, A., Ibrahim, J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics 35, 2084–2092 (2019).
Szabo, P. A. et al. Single-cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat. Commun. 10, 4706 (2019).
Shukla, S. A. et al. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat. Biotechnol. 33, 1152–1158 (2015).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949.e16 (2017).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Reynisson, B., Alvarez, B., Paul, S., Peters, B. & Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 57, 289–300 (1995).
Acknowledgements
We would like to thank A. Taylor, R. Beroukhim, Y. Li, A. Cherniack, M. Kaufman and A. Arina for their guidance and advice regarding genomic, transcriptomic and immunologic analyses. This work was supported by the Ludwig Cancer Research Foundation (S.P.P. and R.R.W.), a Career Development Award from the LUNGevity Foundation (S.P.P.), an Ullman Scholarship in Translational Cancer Immunology from the University of Chicago Comprehensive Cancer Center (UCCCC) (S.P.P.), a Cancer Spotlight Grant (S.P.P.) from the UCCCC, a Fight Against Cancer Grant from the United-4 A Cure Foundation (S.P.P.), an NIH NCI-SOAR Grant 1R25CA240134-01 (L.F.S.), NSF2016307 (M.C.), R01 grants GM126553 and HG011883 (M.C.), a Sloan Research Foundation fellowship (M.C.) and an NCI CCSG grant (Y.Z.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.F.S. and S.P.P. conceived the study. L.F.S., W.K., M.C., Y.Z., R.H., S.I.G., W.T.T., C.M.L., K.B.P., P.C., S.M., A.N.H., B.C., E.E.V., C.M.B., J.D.P., S.J.C. and S.P.P. acquired the data. L.F.S., C.A.M., W.K., M.C., M.D., T.F.G. and S.P.P. developed the methods. L.F.S., C.A.M., W.K., M.C., Y.Z., A.N.H., M.D., T.F.G. and S.P.P. performed the investigation. L.F.S. and S.P.P. prepared the visualization. L.F.S., C.M.B., J.D.P., R.R.W., S.J.C. and S.P.P. acquired funding. R.R.W., S.J.C. and S.P.P. supervised the work. L.F.S. and S.P.P initially wrote the manuscript. All authors contributed to the final editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.M. is an advisor for Olympus America, Medtronic, Johnson & Johnson, ERBE, Boston Scientific, Cook and Pinnacle Biologics. E.E.V. has served as an advisor for AbbVie, AstraZeneca, BeiGene, BioNTech, Eli Lilly, ED Serono, Genentech/Roche, GlaxoSmithKline, Merck and Novartis. C.M.B. reports serving in a consulting or advisory role for AbbVie, AstraZeneca, Genentech, Pfizer, Seattle Genetics and Takeda. J.D.P. serves as an advisor for AstraZeneca, Takeda and Genentech, and receives research funding from Bristol Myers Squibb (institution). M.D. reports personal fees from Roche Sequencing Solutions; grants and personal fees from Astra-Zeneca, Illumina and Genentech; personal fees from Novartis, Gritstone Oncology, BioNTech and Boehringer Ingelheim; grants from Varian Medical Systems; other support from CiberMed Foresight Diagnostics; a patent for ctDNA detection issued and licensed to Roche; and patents for ctDNA detection pending and licensed to Foresight Diagnostics. R.R.W. reports having stock and other ownership interests in Boost Therapeutics, ImmVira, Reflexion Pharmaceuticals, Coordination Pharmaceuticals, Magi Therapeutics and Oncosenescence; serving in a consulting or advisory role for Aettis, AstraZeneca, Coordination Pharmaceuticals, Genus, Merck Serono, NanoProteagen, NKMax America, Shuttle Pharmaceuticals and Highlight Therapeutics, S.L. holds research grants with Varian and Regeneron, and receives compensation (including cost of travel and accommodations and other expenses) from AstraZeneca, Boehringer Ingelheim and Merck Serono. S.J.C. reports participating in the advisory boards for Genentech and AstraZeneca; receiving research support from Bristol Myers Squibb, Merck, EMD Serono and AstraZeneca; and having his spouse who works for Astellas. S.P.P. has patents outside of the submitted work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Elise Alspach and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Consort diagram of patient selection and data analytical framework.
a) Patient selection for clinical and genomic analyses. Patients were excluded based on manual pathologic review and inspection of genomic results. (b) Schematic of genomic and transcriptomic analysis workflow.
Extended Data Fig. 2 OncoPrint of COSINR patient cohort.
OncoPrint plot of tumors which successfully underwent whole exome sequencing (n = 40 samples). Paired samples are adjacent with individual patients separated by larger white breaks. Clinical and pathological data are displayed above variants. Dominant mutational processes reflect the mutational signature etiology with the largest contribution to the sample’s overall mutational profile. Percentages reflect the prevalence of gene alterations in the pre-treatment samples. Bar graphs on the right of the plot reflect the total number of gene alterations across all samples.
Extended Data Fig. 3 Baseline clinical characteristics of COSINR cohort.
(a) Association of clinicopathologic variables with progression-free (top) and overall survival (bottom). (b) Progression-free and overall survival of entire cohort by treatment arm. Dotted vertical lines represent median survival; two-sided Log-rank test. (c) Progression-free and overall survival by treatment arm in the subset of patients used for molecular analysis; two-sided Log-rank test; dotted lines represent median survival (n = 22 patients).
Extended Data Fig. 4 Changes in genomic and transcriptomic features on therapy.
(a) Clonal evolution of somatic mutations on treatment. Each box corresponds to an individual patient. Axes indicate the variant allele fractions (VAFs) of each mutation (x-axis: pre-treatment; y-axis: on-treatment); oncogenic mutations highlighted in blue; (n = 18 patients). (b) Differences in on-treatment density of (TTF1+/CK5+) tumor cells as determined by mIF between treatment arms; (SBRT n = 6 patients, SBRT+Ipi/Nivo n = 6 patients); two-sided Wilcoxon test. The top and bottom edges represent the 1st and 3rd quartiles, respectively; the center line represents the median; whiskers extend to the farthest data points which do not represent outliers (within 1.5x the interquartile range); outliers are plotted as points above and below the box-and-whisker plot. (c) On treatment changes in purity and ploidy; two-sided paired Wilcoxon test. Patient #12 was excluded from the SBRT+ipi/nivo group because the tumor purity could not accurately be determined for the on-treatment sample (SBRT+Ipi/Nivo n = 7 patients, SBRT n = 10 patients). Boxplot elements are defined in the legend of panel b. (d) Changes in ssGSEA Hallmark pathway scores on-treatment (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); two-sided paired Wilcoxon P<0.05 are highlighted in blue. (e) Plots illustrating patient-level changes in ssGSEA Hallmark pathways determined to be significantly differentially changed between treatment arms (see Fig. 2d); (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); two-sided paired Wilcoxon test. Box plot elements are defined in the legend of panel b. (f) Changes in ESTIMATE stromal score on treatment in each treatment arm; (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); two-sided paired Wilcoxon test. Box plot elements are defined in the legend of panel b.
Extended Data Fig. 5 Changes in T cell landscape during treatment.
(a) Balance in baseline immune cell signatures across COSINR treatment arms using the four xCell signature matrices. Dashed line indicates SBRT = SBRT+Ipi/Nivo. All T-cell associated signatures and any signature with two-sided Wilcoxon P<0.05 are labeled. Blue points are P<0.05 (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); paired two-sided Wilcoxon signed-rank test. (b) Changes in CD8+ T cell populations using the 4 xCell signatures; (SBRT n = 8 patients, SBRT + Ipi/Nivo n = 7 patients). Box plot elements are defined in the legend of Extended Data Figure 4b. (c) Changes in TCR richness and evenness (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); two-sided paired Wilcoxon test. Box plot elements are defined in the legend of Extended Data Figure 4b. (d) Evolution of TCR clonotypes at a per-patient level; horizontal dotted lines represent the median number of novel TCRs per treatment group (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients). (e) Two-sided Spearman correlation between pre-treatment (left) and on-treatment (right) CD8+ T cell populations and the number (richness) of TCRs (n = 15 patients).
Extended Data Fig. 6 Integrative T cell characterization using RNA-seq and immunofluorescence.
(a) Correlation between xCell immune cell type scores and CD8+ T cell density as determined by mIF; two-sided Spearman correlation (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients). (b) Correlation between change in effector T cell IFNγ signature and change in CD8+ T cell density; two-sided Spearman correlation (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients). (c) Changes in CD8+ T cell density as determined by mIF by treatment arm; (SBRT n = 6 patients, SBRT + Ipi/Nivo n = 6 patients); two-sided Wilcoxon test. Box plot elements are defined in the legend of Extended Data Figure 4b. (d) Change in naïve T cell gene expression signature by treatment arm; (SBRT n = 8 patients, SBRT+Ipi/Nivo n = 7 patients); two-sided Wilcoxon test. Box plot elements are defined in Extended Data Figure 4b. (e) Change in the fraction of PD-L1-positive tumor and stromal cells by mIF across treatment arms; (SBRT n = 6 patients, SBRT+Ipi/Nivo n = 6 patients); two-sided Wilcoxon test. Box plot elements are defined in the legend of Extended Data Figure 4b.
Extended Data Fig. 7 Association between immunotherapy biomarkers and survival.
Association of pre-treatment (a) effector T cell IFNγ signature (n = 15 patients), (b) TMB (n = 22 patients), (c) PD-L1 expression (n = 34 patients), (d) neoantigen count (n = 18 patients), and (e) aneuploidy score (n = 22 patients) with progression-free (left) and overall survival (right). Variables were split at the median; two-sided Log-rank test.
Extended Data Fig. 8 Aneuploidy biomarker development in mNSCLC.
(a) PFS for COSINR patients with high aneuploidy score (AS, ≥median) (left) and low AS (<median, right) tumors; two-sided Log-rank test. (b) Scatter plot of AS and tumor purity (COSINR, n = 22 patients). (c) Comparison of number of pre-treatment organ sites by COSINR treatment arm and aneuploidy group (n = 22 patients); two-sided Wilcoxon test. Box plot elements are defined in the legend of Extended Data Figure 4b. (d) Association of clinical and pathological factors with overall survival in UC cohort (n = 58 patients). Variables tested were age, sex (M vs. F), presence of brain or liver metastases, smoking status (ever vs. never), PD-L1 expression (≥50% vs. <50%), histology (adenocarcinoma vs. other), number of disease sites, TMB, ECOG (0-1 vs. 2-3), and ICB paradigm (monotherapy vs. combination therapy). Variables significantly associated with OS are highlighted in blue; two-sided Wald test. (e) Distribution of AS in COSINR (n = 22 patients), UC (n = 58 patients), and TCGA (n = 500 patients) cohorts; dotted line represents high AS threshold (0.42). (f) Selection of optimal high AS threshold based on leave-one-out cross validation analysis; bars: 95% confidence interval; points: mean. Grey lines outline optimal AS threshold (0.42) (n = 58 patients). (g) Differences in OS in high AS (≥0.42) and low AS (<0.42) groups in UC validation cohort using the derived optimal threshold; two-sided Log-rank test. Dotted maroon and yellow lines represent subdivisions of the RT/ICB treatment group into patients treated with concurrent (maroon) or sequential (yellow) RT + ICB. (h) Application of the derived optimal threshold (0.42) to the COSINR cohort (OS); two-sided Log-rank test.
Supplementary information
Supplementary Tables 1–20
These tables contain the raw source data to replicate all analyses in the manuscript, analysis of clinicogenomic confounders and information about immunofluorescence antibodies.
Source data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 3
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Fig. 5
Statistical Source Data
Source Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 2
Statistical Source Data
Source Data Extended Data Fig. 3
Statistical Source Data
Source Data Extended Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig. 5
Statistical Source Data
Source Data Extended Data Fig. 6
Statistical Source Data
Source Data Extended Data Fig. 7
Statistical Source Data
Source Data Extended Data Fig. 8
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spurr, L.F., Martinez, C.A., Kang, W. et al. Highly aneuploid non-small cell lung cancer shows enhanced responsiveness to concurrent radiation and immune checkpoint blockade. Nat Cancer 3, 1498–1512 (2022). https://doi.org/10.1038/s43018-022-00467-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-022-00467-x
This article is cited by
-
Aneuploid serves as a prognostic marker and favors immunosuppressive microenvironment in ovarian cancer
Journal of Ovarian Research (2024)
-
Exploiting tumor aneuploidy as a biomarker and therapeutic target in patients treated with immune checkpoint blockade
npj Precision Oncology (2024)
-
An international phase II trial and immune profiling of SBRT and atezolizumab in advanced pretreated colorectal cancer
Molecular Cancer (2024)
-
Exploiting RIG-I-like receptor pathway for cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
Systemic immune modulation by stereotactic radiotherapy in early-stage lung cancer
npj Precision Oncology (2023)