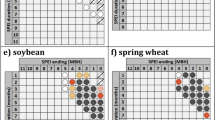
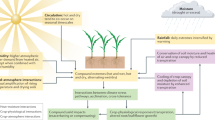
Abstract
Drought limits crop productivity and threatens global food security, with moderate drought stress — when crops grow at a reduced rate — commonly experienced. Increasing plant tolerance to moderate drought is a key target for adaptation and management, but efforts to understand and increase drought tolerance often focus on more extreme drought that causes complete crop failure and only consider crop genetics. In this Review, we discuss the influence of moderate drought on crop productivity and the role of physiological traits in drought tolerance and adaptation. Traits related to crop water use, water capture, water availability, transpiration efficiency and phenology impact drought adaptation, but their overall effect varies situationally. For example, early restrictions in transpiration, higher transpiration efficiency or altered tillering increase water availability during grain filling and can double yield in some drought scenarios. However, these same traits under less severe drought scenarios can also lead to yield penalties. To assess when and under what conditions traits will be beneficial, crop models are used to integrate the effects of genetics, the environment and management, estimating the expected yield responses under these combinations of scenarios and traits. More robust characterization of moderate drought tolerance and better integration between plant genetic information and modelling will enable the local selection of crop varieties suited to the expected drought scenarios.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sinclair, T. R., Tanner, C. B. & Bennett, J. M. Water-use efficiency in crop production. BioScience 34, 36–40 (1984).
Lobell, D. B. et al. Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344, 516–519 (2014).
Yang, Y., Liu, D. L., Anwar, M. R., Zuo, H. & Yang, Y. Impact of future climate change on wheat production in relation to plant-available water capacity in a semiarid environment. Theor. Appl. Climatol. 115, 391–410 (2014).
Zaman-Allah, M., Jenkinson, D. M. & Vadez, V. A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J. Exp. Bot. 62, 4239–4252 (2011).
Collins B., Chapman S., Hammer G. & Chenu K. Limiting transpiration rate in high evaporative demand conditions to improve Australian wheat productivity in silico. Plants 3 https://doi.org/10.1093/insilicoplants/diab006 (2021).
Vadez, V., Kholova, J., Medina, S., Kakkera, A. & Anderberg, H. Transpiration efficiency: new insights into an old story. J. Exp. Bot. 65, 6141–6153 (2014).
Chenu, K. et al. Contribution of crop models to adaptation in wheat. Trends Plant. Sci. 22, 472–490 (2017).
Messina, C. D., Podlich, D., Dong, Z., Samples, M. & Cooper, M. Yield-trait performance landscapes: from theory to application in breeding maize for drought tolerance. J. Exp. Bot. 62, 855–868 (2011).
Kholova, J., McLean, G., Vadez, V., Craufurd, P. & Hammer, G. L. Drought stress characterization of post-rainy season (rabi) sorghum in India. Field Crop. Res. 141, 38–46 (2013).
Chenu, K., Deihimfard, R. & Chapman, S. C. Large-scale characterization of drought pattern: a continent-wide modelling approach applied to the Australian wheatbelt spatial and temporal trends. N. Phytol. 198, 801–820 (2013).
Harrison, M., Tardieu, F., Dong, Z., Messina, C. & Hammer, G. Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob. Change Biol. 20, 867–878 (2014).
Hajjarpoor, A. et al. Environmental characterization and yield gap analysis to tackle genotype-by-environment-by-management interactions and map region-specific agronomic and breeding targets in groundnut. Field Crops Res. 267, 108160 (2021).
Bhatnagar-Mathur, P., Vadez, V. & Sharma, K. K. Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant. Cell Rep. 27, 411–424 (2008).
Lawlor, D. W. Genetic engineering to improve plant performance under drought: physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 64, 83–108 (2013).
Tardieu, F. Any trait or trait-related allele can confer drought tolerance: just design the right drought scenario. J. Exp. Bot. 63, 25–31 (2012).
Rodriguez, D. Predicting optimum crop designs using crop models and seasonal climate forecasts. Sci. Rep. 8, 2231 (2018).
Hajjarpoor, A. How process-based modeling can help plant breeding deal with G × E × M interactions. Field Crops Res. 283, 108554 (2022).
Vadez, V. et al. Adaptation of grain legumes to climate change: a review. Agron. For. Sustain. Dev. 32, 31–44 (2012).
Koehler, T. et al. Going underground: soil hydraulic properties impacting maize responsiveness to water deficit. Plant. Soil. 478, 43–58 (2022).
Sinclair, T. & Ludlow, M. Influence of soil water supply on the plant water balance of four tropical grain legumes. Funct. Plant. Biol. 13, 329 (1986).
Pellegrineschi, A. et al. Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47, 493–500 (2004).
Juenger, T. E. & Verslues, P. E. Time for a drought experiment: do you know your plants’ water status? Plant. Cell 35, 10–23 (2023).
Craufurd, P. Q., Flower, D. J. & Peacock, J. M. Effect of heat and drought stress on sorghum (Sorghum bicolor). I. Panicle development and leaf appearance. Ex. Agric. 29, 61–76 (1993).
Marrou, H., Vadez, V. & Sinclair, T. R. Plant survival of drought during establishment: an interspecific comparison of five grain legumes. Crop. Sci. 55, 1264–1273 (2015).
Passioura, J. Grain yield, harvest index and water use of wheat. J. Aust. Inst. Agric. Sci. 43, 117–120 (1977).
Vadez, V., Kholova, J., Yadav, R. S. & Hash, C. T. Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R. Br.) are critical for grain yield under terminal drought. Plant. Soil. 371, 447–462 (2013).
Borrell, A. K. et al. Stay‐green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. N. Phytol. 203, 817–830 (2014).
Messina, C. D. et al. Limited-transpiration trait may increase maize drought tolerance in the US corn belt. Agron. J. 107, 1978–1986 (2015).
Borrell, A. K. et al. Genetic modification of PIN genes induces causal mechanisms of stay-green drought adaptation phenotype. J. Exp. Bot. 73, 6711–6726 (2022).
Kashiwagi, J. et al. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 146, 213–222 (2005).
Wang, Y. et al. Reducing basal nitrogen rate to improve maize seedling growth, water and nitrogen use efficiencies under drought stress by optimizing root morphology and distribution. Agric. Water Manag. 212, 328–337 (2019).
Soltani, A., Ghassemi-Golezani, K., Khooie, F. R. & Moghaddam, M. A simple model for chickpea growth and yield. Field Crop. Res. 62, 213–224 (1999).
Sujariya, S., Jongdee, B. & Fukai, S. Estimation of flowering time and its effect on grain yield of photoperiod sensitive varieties in rainfed lowland rice in northeast Thailand. Field Crop. Res. 302, 109075 (2023).
Monkham, T. et al. Genotypic variation in grain yield and flowering pattern in terminal and intermittent drought screening methods in rainfed lowland rice. Field Crop. Res. 175, 26–36 (2015).
Bidinger, F., Mahalakshmi, V. & Rao, G. Assessment of drought resistance in pearl-millet [Pennisetum americanum (l) Leeke]. 1. Factors affecting yields under stress. Aust. J. Agric. Res. 38, 37–48 (1987).
Kouressy, M., Dingkuhn, M., Vaksmann, M. & Heinemann, A. B. Adaptation to diverse semi-arid environments of sorghum genotypes having different plant type and sensitivity to photoperiod. Agric. For. Meteorol. 148, 357–371 (2008).
Nord, E. A. & Lynch, J. P. Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant. Cell Environ. 31, 1432–1441 (2008).
Beggi, F., Falalou, H., Buerkert, A. & Vadez, V. Tolerant pearl millet (Pennisetum glaucum (L.) R. Br.) varieties to low soil P have higher transpiration efficiency and lower flowering delay than sensitive ones. Plant. Soil. 389, 89–108 (2015).
Costa, W. A. J. M. D., Dennett, M. D., Ratnaweera, U. & Nyalemegbe, K. Effects of different water regimes on field-grown determinate and indeterminate faba bean (Vicia faba L.). I. Canopy growth and biomass production. Field Crop. Res. 49, 83–93 (1997).
Van Oosterom, E. J., Weltzien, E., Yadav, O. P. & Bidinger, F. R. Grain yield components of pearl millet under optimum conditions can be used to identify germplasm with adaptation to arid zones. Field Crop. Res. 96, 407–421 (2006).
Dodsworth, S. A diverse and intricate signalling network regulates stem cell fate in the shoot apical meristem. Dev. Biol. 336, 1–9 (2009).
Chang, W., Guo, Y., Zhang, H., Liu, X. & Guo, L. Same actor in different stages: genes in shoot apical meristem maintenance and floral meristem determinacy in Arabidopsis. Front. Ecol. Evol. 8, 89 (2020).
Laux, T., Mayer, K. F. X., Berger, J. & Jürgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122, 87–96 (1996).
Schoof, H. et al. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644 (2000).
Brambilla, V., Gomez-Ariza, J., Cerise, M. & Fornara, F. The importance of being on time: regulatory networks controlling photoperiodic flowering in cereals. Front. Plant. Sci. 8, 665 (2017).
Gomez-Ariza, J. et al. A transcription factor coordinating internode elongation and photoperiodic signals in rice. Nat. Plants 5, 358–362 (2019).
Vicentini, G. et al. Environmental control of rice flowering time. Plant. Commun. 4, 100610 (2023).
Kim, S.-R. et al. Loss-of-function alleles of heading date 1 (Hd1) are associated with adaptation of temperate japonica rice plants to the tropical region. Front. Plant. Sci. 9, 1827 (2018).
Komiya, R., Yokoi, S. & Shimamoto, K. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 136, 3443–3450 (2009).
Liang, Y. et al. Zm MADS 69 functions as a flowering activator through the ZmRap2.7‐ZCN 8 regulatory module and contributes to maize flowering time adaptation. N. Phytol. 221, 2335–2347 (2019).
Campoli, C., Shtaya, M., Davis, S. J. & von Korff, M. Expression conservation within the circadian clock of a monocot: natural variation at barley Ppd-H1 affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant. Biol. 12, 97 (2012).
Du, H. et al. Integrative regulation of drought escape through ABA-dependent and -independent pathways in rice. Mol. Plant. 11, 584–597 (2018).
Galbiati, F. et al. Hd3a, RFT1 and Ehd1 integrate photoperiodic and drought stress signals to delay the floral transition in rice: integration of drought and photoperiod in rice. Plant Cell Environ. 39, 1982–1993 (2016).
Zhang, L., Marguerit, E., Rossdeutsch, L., Ollat, N. & Gambetta, G. A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. And. Exp. Plant. Physiol. 28, 143–157 (2016).
Lin, F. et al. GF14f gene is negatively associated with yield and grain chalkiness under rice ratooning. Front. Plant. Sci. 14, 1112146 (2023).
Allen, R. G., Pereira, L. S., Raes, D. & Smith, M. Crop Evapotranspiration — Guidelines for Computing Crop Water Requirements — FAO Irrigation and Drainage Paper 56 (Food and Agriculture Organization of the United Nations, 1998).
Halilou, O. et al. Determination of coefficient defining leaf area development in different genotypes, plant types and planting densities in peanut (Arachis hypogeae L.). Field Crop. Res. 199, 42–51 (2016).
Vadez, V. et al. Transpiration efficiency: insights from comparisons of C-4 cereal species. J. Exp. Bot. 72, 5221–5234 (2021).
Reymond, M., Muller, B., Leonardi, A., Charcosset, A. & Tardieu, F. Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant. Physiol. 131, 664–675 (2003).
Parent, B. et al. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant. Physiol. 149, 2000–2012 (2009).
Ehlert, C., Maurel, C., Tardieu, F. & Simonneau, T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant. Physiol. 150, 1093–1104 (2009).
Pantin, F., Simonneau, T., Rolland, G., Dauzat, M. & Muller, B. Control of leaf expansion: a developmental switch from metabolics to hydraulics. Plant. Physiol. 156, 803–815 (2011).
Martre, P. et al. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant. Physiol. 130, 2101–2110 (2002).
Ding, L. et al. Modification of the expression of the aquaporin ZmPIP2;5 affects water relations and plant growth. Plant. Physiol. 182, 2154–2165 (2020).
Wu, Y. & Cosgrove, D. J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 51, 1543–1553 (2000).
Welcker, C. et al. A common genetic determinism for sensitivities to soil water deficit and evaporative demand: meta-analysis of quantitative trait loci and introgression lines of maize. Plant. Physiol. 157, 718–729 (2011).
Dignat, G., Welcker, C., Sawkins, M., Ribaut, J. M. & Tardieu, F. The growths of leaves, shoots, roots and reproductive organs partly share their genetic control in maize plants: common QTLs for growth of different organs. Plant. Cell Env. 36, 1105–1119 (2013).
Cairns, J. E. et al. Investigating early vigour in upland rice (Oryza sativa L.): part II. Identification of QTLs controlling early vigour under greenhouse and field conditions. Field Crop. Res. 113, 207–217 (2009).
Condon, A. G., Richards, R. A., Rebetzke, G. J. & Farquhar, G. D. Breeding for high water-use efficiency. J. Exp. Bot. 55, 2447–2460 (2004).
Rebetzke, G. & Richards, R. Genetic improvement of early vigour in wheat. Aust J. Agric. Res. 50, 291–301 (1999).
Sivasakthi, K. et al. Plant vigour QTLs co-map with an earlier reported QTL hotspot for drought tolerance while water saving QTLs map in other regions of the chickpea genome. BMC Plant Biol. 18, 29 (2018).
ter Steege, M. W., den Ouden, F. M., Lambers, H., Stam, P. & Peeters, A. J. M. Genetic and physiological architecture of early vigor in Aegilops tauschii, the D-genome donor of hexaploid wheat. A quantitative trait loci analysis. Plant. Physiol. 139, 1078–1094 (2005).
Rebolledo, M. C. et al. Phenotypic and genetic dissection of component traits for early vigour in rice using plant growth modelling, sugar content analyses and association mapping. EXBOTJ 66, 5555–5566 (2015).
Affortit, P. et al. Physiological and genetic control of transpiration efficiency in African rice, Oryza glaberrima Steud. J. Exp. Bot. 73, 5279–5293 (2022).
Vukasovic, S. et al. Dissecting the genetics of early vigour to design drought-adapted wheat. Front. Plant. Sci. 12, 754439 (2022).
Pilloni, R. Agronomical and Physiological Study of the Response of Sorghum and Pearl Millet Crops to Higher Sowing Density in the Semi-arid Tropics. Assessemnt of the Opportunity for Sustainable Intensification and Consequence for the Transpiration Response to Evaporative Demand of the Crops. PhD thesis, Univ. de Montpellier (2022).
Singh, V., Van Oosterom, E. J., Jordan, D. R. & Hammer, G. L. Genetic control of nodal root angle in sorghum and its implications on water extraction. Eur. J. Agron. 42, 3–10 (2012).
Grondin, A. et al. Aquaporins are main contributors to root hydraulic conductivity in pearl millet [Pennisetum glaucum (L) R. Br.]. PLoS ONE 15, e0233481 (2020).
Kashiwagi, J., Krishnamurthy, L., Crouch, J. H. & Serraj, R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop. Res. 95, 171–181 (2006).
Manschadi, A. M., Christopher, J., deVoil, P. & Hammer, G. L. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct. Plant. Biol. 33, 823 (2006).
Lynch, J. P. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Botany 112, 347–357 (2013).
Klein, S. P., Schneider, H. M., Perkins, A. C., Brown, K. M. & Lynch, J. P. Multiple integrated root phenotypes are associated with improved drought tolerance. Plant Physiol. 83, 1011–1025 (2020).
Lynch, J. P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 69, 3279–3292 (2018).
Schneider, R. et al. Restoring soil health to reduce irrigation demand and buffer the impacts of drought. Front. Agric. Sci. Eng. 7, 339–346 (2020).
Menge, D. M., Kano‐Nakata, M., Yamauchi, A., Suralta, R. R. & Makihara, D. Root and shoot responses of upland new rice for Africa varieties to fluctuating soil moisture conditions as affected by different levels of nitrogen fertilization. J. Agron. Crop. Sci. 206, 322–337 (2020).
Uga, Y., Okuno, K. & Yano, M. Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 62, 2485–2494 (2011).
Guseman, J. M., Webb, K., Srinivasan, C. & Dardick, C. DRO 1 influences root system architecture in Arabidopsis and Prunus species. Plant. J. 89, 1093–1105 (2017).
Feng, X. et al. ABA‐inducible DEEPER ROOTING 1 improves adaptation of maize to water deficiency. Plant. Biotechnol. J. 20, 2077–2088 (2022).
Ogura, T. et al. Root system depth in Arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 178, 400–412.e16 (2019).
Kirschner, G. K. et al. ENHANCED GRAVITROPISM 2 encodes a STERILE ALPHA MOTIF-containing protein that controls root growth angle in barley and wheat. Proc. Natl Acad. Sci. USA 118, e2101526118 (2021).
Fusi, R. et al. Root angle is controlled by EGT1 in cereal crops employing an antigravitropic mechanism. Proc. Natl Acad. Sci. USA 119, e2201350119 (2022).
Schneider, H. M. et al. Transcription factor bHLH121 regulates root cortical aerenchyma formation in maize. Proc. Natl Acad. Sci. USA 120, e2219668120 (2023).
Kawai, T. et al. WUSCHEL-related homeobox family genes in rice control lateral root primordium size. Proc. Natl Acad. Sci. USA 119, e2101846119 (2022).
Scharwies, J. D. & Dinneny, J. R. Water transport, perception, and response in plants. J. Plant. Res. 132, 311–324 (2019).
Maurel, C. & Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 6, 744–749 (2020).
Rishmawi, L. et al. Natural variation of maize root hydraulic architecture underlies highly diverse water uptake capacities. Plant. Physiol. 192, 2404–2418 (2023).
Maurel, C., Simonneau, T. & Sutka, M. The significance of roots as hydraulic rheostats. J. Exp. Bot. 61, 3191–3198 (2010).
Tsuda, M. & Tyree, M. T. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J. Exp. Bot. 51, 823–828 (2000).
Tharanya, M. et al. Pearl millet (Pennisetum glaucum) contrasting for the transpiration response to vapour pressure deficit also differ in their dependence on the symplastic and apoplastic water transport pathways. Funct. Plant. Biol. 45, 719–736 (2018).
Calvo‐Polanco, M. et al. Physiological roles of Casparian strips and suberin in the transport of water and solutes. N. Phytol. 232, 2295–2307 (2021).
Burridge, J. D., Grondin, A. & Vadez, V. Optimizing crop water use for drought and climate change adaptation requires a multi-scale approach. Front. Plant Sci. 13, 824720 (2022).
Ding, L. et al. The plasma membrane aquaporin ZmPIP2;5 enhances the sensitivity of stomatal closure to water deficit. Plant. Cell Environ. 45, 1146–1156 (2022).
Augstein, F. & Carlsbecker, A. Getting to the roots: a developmental genetic view of root anatomy and function from arabidopsis to lycophytes. Front. Plant. Sci. 9, 1410 (2018).
Ramachandran, P., Wang, G., Augstein, F., de Vries, J. & Carlsbecker, A. Continuous root xylem formation and vascular acclimation to water deficit involves endodermal ABA signalling via miR165. Development 145, dev159202 (2018).
Richards, R. & Passioura, J. A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain-yield in rain-fed environments. Aust. J. Agric. Res. 40, 943–950 (1989).
Passioura, J. B. The meaning of matric potential. J. Exp. Bot. 31, 1161–1169 (1980).
Carminati, A., Vetterlein, D., Weller, U., Vogel, H.-J. & Oswald, S. E. When roots lose contact. Vadose Zone J. 8, 805–809 (2009).
Cai, G., Ahmed, M. A., Abdalla, M. & Carminati, A. Root hydraulic phenotypes impacting water uptake in drying soils. Plant. Cell Environ. 45, 650–663 (2022).
Affortit, P. et al. Keep in touch: the soil–root hydraulic continuum and its role in drought resistance in crops. J. Exp. Bot. erad312 https://doi.org/10.1093/jxb/erad312 (2023).
Carminati, A. et al. Root hairs enable high transpiration rates in drying soils. N. Phytol. 216, 771–781 (2017).
Marin, M. et al. Significance of root hairs for plant performance under contrasting field conditions and water deficit. Ann. Botany 128, 1–16 (2021).
Duddek, P. et al. The impact of drought-induced root and root hair shrinkage on root–soil contact. Plant. Physiol. 189, 1232–1236 (2022).
Kohli, P. S., Maurya, K., Thakur, J. K., Bhosale, R. & Giri, J. Significance of root hairs in developing stress‐resilient plants for sustainable crop production. Plant. Cell Environ. 45, 677–694 (2022).
Sandhu, N. et al. Traits and QTLs for development of dry direct-seeded rainfed rice varieties. J. Exp. Bot. 66, 225–244 (2015).
Horn, R., Wingen, L. U., Snape, J. W. & Dolan, L. Mapping of quantitative trait loci for root hair length in wheat identifies loci that co-locate with loci for yield components. J. Exp. Bot. 67, 4535–4543 (2016).
Duan, S. et al. A natural non‐synonymous single nucleotide polymorphism in GmbHLH113 negates its inhibitory effect on root hair elongation in soybean. Plant J. 115, 742–757 (2023).
Moreno-Espíndola, I. P., Rivera-Becerril, F., de Jesús Ferrara-Guerrero, M. & De León-González, F. Role of root-hairs and hyphae in adhesion of sand particles. Soil. Biol. Biochem. 39, 2520–2526 (2007).
North, G. B. & Nobel, P. S. Root–soil contact for the desert succulent Agave deserti in wet and drying soil. N. Phytol. 135, 21–29 (1997).
Rabbi, S. M. F., Tighe, M. K., Knox, O. & Young, I. M. The impact of carbon addition on the organisation of rhizosheath of chickpea. Sci. Rep. 8, 18028 (2018).
Liu, T. et al. Rhizosheath formation and involvement in foxtail millet (Setaria italica) root growth under drought stress. J. Integr. Plant. Biol. 61, 449–462 (2019).
de la Fuente Cantó, C. et al. Genetic control of rhizosheath formation in pearl millet. Sci. Rep. 12, 9205 (2022).
Pauwels, R., Graefe, J. & Bitterlich, M. An arbuscular mycorrhizal fungus alters soil water retention and hydraulic conductivity in a soil texture specific way. Mycorrhiza 33, 165–179 (2023).
Sadras, V. O. & Angus, J. F. Benchmarking water-use efficiency of rainfed wheat in dry environments. Aust. J. Agric. Res. 57, 847–856 (2006).
Kirkegaard, J. A., Lilley, J. M., Howe, G. N. & Graham, J. M. Impact of subsoil water use on wheat yield. Aust. J. Agric. Res. 58, 303 (2007).
Slewinski, T. L. Non-structural carbohydrate partitioning in grass stems: a target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 63, 4647–4670 (2012).
Talbert, L. E., Lanning, S. P., Murphy, R. L. & Martin, J. M. Grain fill duration in twelve hard red spring wheat crosses: genetic variation and association with other agronomic traits. Crop. Sci. 41, 1390–1395 (2001).
Gasura, E., Setimela, P., Edema, R., Gibson, P. T., Okori, P. & Tarekegne, A. M. Exploiting grain-filling rate and effective grain-filling duration to improve grain yield of early-maturing maize. Crop Sci. 53, 2295–2303 (1999).
Mwanamwenge, J., Loss, S. P., Siddique, K. H. M. & Cocks, P. S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 11, 1–11 (1999).
Cruz, R. & Otoole, J. Dryland rice response to an irrigation gradient at flowering stage. Agron. J. 76, 178–183 (1984).
Aparna, K., Hash, C. T., Yadav, R. S. & Vadez, V. Seed number and 100-seed weight of pearl millet (Pennisetum glaucum L.) respond differently to low soil moisture in genotypes contrasting for drought tolerance. J. Agron. Crop Sci. 200, 119–131 (2014).
Boyer, J. & Westgate, M. Grain yields with limited water. J. Exp. Bot. 55, 2385–2394 (2004).
Fuad-Hassan, A., Tardieu, F. & Turc, O. Drought-induced changes in anthesis-silking interval are related to silk expansion: a spatio-temporal growth analysis in maize plants subjected to soil water deficit. Plant. Cell Environ. 31, 1349–1360 (2008).
Turc, O., Bouteille, M., Fuad-Hassan, A., Welcker, C. & Tardieu, F. The growth of vegetative and reproductive structures (leaves and silks) respond similarly to hydraulic cues in maize. N. Phytol. 212, 377–388 (2016).
Edmeades, G. O. et al. The role and regulation of the anthesis-silking interval in maize. In: Westgate, M. E., Boote, K. J. (eds) Physiology and modeling kernel set in maize. CSSA special publication no. 29. CSSA, Madison WI, pp. 43–73 (2000).
Fletcher, A. L., Sinclair, T. R. & Allen, L. H. Transpiration responses to vapor pressure deficit in well watered ‘slow-wilting’ and commercial soybean. Environ. Exp. Botany 61, 145–151 (2007).
Gholipoor, M., Prasad, P. V. V., Mutava, R. N. & Sinclair, T. R. Genetic variability of transpiration response to vapor pressure deficit among sorghum genotypes. Field Crop. Res. 119, 85–90 (2010).
Kholova, J. et al. Terminal drought-tolerant pearl millet [Pennisetum glaucum (L.) R. Br.] have high leaf ABA and limit transpiration at high vapour pressure deficit. J. Exp. Bot. 61, 1431–1440 (2010).
Schoppach, R. & Sadok, W. Differential sensitivities of transpiration to evaporative demand and soil water deficit among wheat elite cultivars indicate different strategies for drought tolerance. Environ. Exp. Bot. 84, 1–10 (2012).
Sadok, W. & Sinclair, T. R. Transpiration response of ‘slow-wilting’ and commercial soybean (Glycine max (L.) Merr.) genotypes to three aquaporin inhibitors. J. Exp. Bot. 61, 821–829 (2010).
Reddy, P. S. et al. Molecular cloning and expression analysis of aquaporin genes in pearl millet [Pennisetum glaucum (L) R. Br.] genotypes contrasting in their transpiration response to high vapour pressure deficits. Plant. Sci. 265, 167–176 (2017).
Yang, Z. et al. Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc. Natl Acad. Sci. USA 113, 6791–6796 (2016).
Mega, R. et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 5, 153–159 (2019).
Thompson, A. J. et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant. Physiol. 143, 1905–1917 (2007).
Van Oosterom, E. J., Borrell, A. K., Deifel, K. S. & Hammer, G. L. Does increased leaf appearance rate enhance adaptation to postanthesis drought stress in sorghum? Crop. Sci. 51, 2728–2740 (2011).
Kholov, J. et al. Modelling the effect of plant water use traits on yield and stay-green expression in sorghum. Funct. Plant. Biol. 41, 1019–1034 (2014).
Christopher, J. T., Christopher, M. J., Borrell, A. K., Fletcher, S. & Chenu, K. Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. J. Exp. Bot. 67, 5159–5172 (2016).
Trachsel, S. et al. Identification of QTL for early vigor and stay-green conferring tolerance to drought in two connected advanced backcross populations in tropical maize (Zea mays L.). PLoS ONE 11, e0149636 (2016).
Condon, A. G., Richards, R. A., Rebetzke, G. J. & Farquhar, G. D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 42, 122–131 (2002).
Fletcher, A., Christopher, J., Hunter, M., Rebetzke, G. & Chenu, K. A low-cost method to rapidly and accurately screen for transpiration efficiency in wheat. Plant. Methods 14, 77 (2018).
Dunn, J. et al. Reduced stomatal density in bread wheat leads to increased water-use efficiency. J. Exp. Bot. 70, 4737–4748 (2019).
Liu, S. & Qin, F. Genetic dissection of maize drought tolerance for trait improvement. Mol. Breed. 41, 8 (2021).
Tardieu, F. Different avenues for progress apply to drought tolerance, water use efficiency and yield in dry areas. Curr. Opin. Biotechnol. 73, 128–134 (2022).
Franks, P. J., Doheny-Adams, T. W., Britton-Harper, Z. J. & Gray, J. E. Increasing water‐use efficiency directly through genetic manipulation of stomatal density. N. Phytol. 207, 188–195 (2015).
Hepworth, C., Doheny-Adams, T., Hunt, L., Cameron, D. D. & Gray, J. E. Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. N. Phytol. 208, 336–341 (2015).
Kumar, S. et al. Rice breeding for yield under drought has selected for longer flag leaves and lower stomatal density. J. Exp. Bot. 72, 4981–4992 (2021).
Kholova, J., Hash, C. T., Kakkera, A., Kocova, M. & Vadez, V. Constitutive water-conserving mechanisms are correlated with the terminal drought tolerance of pearl millet [Pennisetum glaucum (L.) R. Br.]. J. Exp. Bot. 61, 369–377 (2010).
Vadez, V., Krishnamurthy, L., Hash, C. T., Upadhyaya, H. D. & Borrell, A. K. Yield, transpiration efficiency, and water-use variations and their interrelationships in the sorghum reference collection. Crop. Pasture Sci. 62, 645–655 (2011).
Ryan, A. et al. Gravimetric phenotyping of whole plant transpiration responses to atmospheric vapour pressure deficit identifies genotypic variation in water use efficiency. Plant. Sci. 251, 101–109 (2016).
Henry, A., Stuart-Williams, H., Dixit, S., Kumar, A. & Farquhar, G. Stomatal conductance responses to evaporative demand conferred by rice drought-yield quantitative trait locus qDTY12.1. Funct. Plant. Biol. 46, 660 (2019).
Pilloni, R. et al. Higher sowing density of pearl millet increases productivity and water use efficiency in high evaporative demand seasons. Front. Plant Sci. 13, 1035181 (2022).
Yin, X. & Struik, P. C. Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: from leaf biochemistry to canopy physiology and crop ecology. EXBOTJ 66, 6535–6549 (2015).
Perez, R. P. A. et al. Changes in the vertical distribution of leaf area enhanced light interception efficiency in maize over generations of selection. Plant. Cell Env. 42, 2105–2119 (2019).
Coupel-Ledru, A. et al. Reduced nighttime transpiration is a relevant breeding target for high water-use efficiency in grapevine. Proc. Natl Acad. Sci. USA 113, 8963–8968 (2016).
Schoppach, R., Sinclair, T. R. & Sadok, W. Sleep tight and wake-up early: nocturnal transpiration traits to increase wheat drought tolerance in a Mediterranean environment. Funct. Plant. Biol. 47, 1117–1127 (2020).
Hammer, G. L. et al. Crop design for specific adaptation in variable dryland production environments. Crop. Pasture Sci. 65, 614–626 (2014).
Chenu, K. et al. Short-term responses of leaf growth rate to water deficit scale up to whole-plant and crop levels: an integrated modelling approach in maize. Plant. Cell Environ. 31, 378–391 (2008).
Vadez, V., Soltani, A. & Sinclair, T. R. Modelling possible benefits of root related traits to enhance terminal drought adaptation of chickpea. Field Crop. Res. 137, 108–115 (2012).
Vadez, V. et al. Mapping water stress incidence and intensity, optimal plant populations, and cultivar duration for African groundnut productivity enhancement. Front. Plant Sci. 8, 432 (2017).
Chenu, K. et al. Simulating the yield impacts of organ-level quantitative trait loci associated with drought response in maize: a ‘gene-to-phenotype’ modeling approach. Genetics 183, 1507–1523 (2009).
Millet, E. et al. Genomic prediction of maize yield across European environmental conditions. Nat. Genet. 51, 952 (2019).
Cooper, M., Technow, F., Messina, C., Gho, C. & Totir, L. R. Use of crop growth models with whole-genome prediction: application to a maize multienvironment trial. Crop Sci. 56, 2141–2156 (2016).
Tardieu, F. & Parent, B. Predictable ‘meta-mechanisms’ emerge from feedbacks between transpiration and plant growth and cannot be simply deduced from short-term mechanisms: meta-mechanisms in plant water relations. Plant, Cell Environ. 40, 846–857 (2017).
Chenu, K. in Crop Physiology. Applications for Genetic Improvement and Agronomy 2nd edn (eds Sadras, V. O. & Calderini, D. F.) 321–348 (Academic, 2015).
Resende, R. T., Chenu, K., Rasmussen, S. K., Heinemann, A. B. & Fritsche-Neto, R. Editorial: enviromics in plant breeding. Front. Plant Sci 13, 935380 (2022).
Ghanem, M. E., Marrou, H., Soltani, A., Kumar, S. & Sinclair, T. R. Lentil variation in phenology and yield evaluated with a model. Agron. J. 107, 1967–1977 (2015).
Collins, B. & Chenu, K. Improving productivity of Australian wheat by adapting sowing date and genotype phenology to future climate. Clim. Risk Manag. 32, 100300 (2021).
Rahimi-Moghaddam, S., Deihimfard, R., Nazari, M. R., Mohammadi-Ahmadmahmoudi, E. & Chenu, K. Understanding wheat growth and the seasonal climatic characteristics of major drought patterns occurring in cold dryland environments from Iran. Eur. J. Agron. 145, 126772 (2023).
Cooper, M. & Messina, C. D. Can we harness ‘enviromics’ to accelerate crop improvement by integrating breeding and agronomy? Front. Plant Sci. 12, 735143 (2021).
Pauli, D. et al. The quest for understanding phenotypic variation via integrated approaches in the field environment. Plant Physiol. 172, 622–634 (2016).
Schwalbert, R. et al. Mid-season county-level corn yield forecast for US corn belt integrating satellite imagery and weather variables. Crop Sci. 60, 739–750 (2020).
Smith, D. T., Potgieter, A. B. & Chapman, S. C. Scaling up high-throughput phenotyping for abiotic stress selection in the field. Theor. Appl. Genet. 134, 1845–1866 (2021).
Yang, Y. et al. High-resolution spatially explicit land surface model calibration using field-scale satellite-based daily evapotranspiration product. J. Hydrol. 596, 125730 (2021).
Martre, P., He, J., Le Gouis, J. & Semenov, M. A. In silico system analysis of physiological traits determining grain yield and protein concentration for wheat as influenced by climate and crop management. J. Exp. Bot. 66, 3581–3598 (2015).
Casadebaig, P. et al. Assessment of the potential impacts of wheat plant traits across environments by combining crop modeling and global sensitivity analysis. PLoS ONE 11, 1–27 (2016).
Bustos-Korts, D. et al. Combining crop growth modeling and statistical genetic modeling to evaluate phenotyping strategies. Front. Plant Sci. 10, 1491 (2019).
Sinclair, T. R., Hammer, G. L. & Van Oosterom, E. J. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Funct. Plant. Biol. 32, 945 (2005).
Sinclair, T. R., Messina, C. D., Beatty, A. & Samples, M. Assessment across the United States of the benefits of altered soybean drought traits. Agron. J. 102, 475–482 (2010).
Flohr, B. M., Hunt, J. R., Kirkegaard, J. A. & Evans, J. R. Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crop Res. 209, 108–119 (2017).
Flohr, B. M. et al. Fast winter wheat phenology can stabilise flowering date and maximise grain yield in semi-arid Mediterranean and temperate environments. Field Crop Res. 223, 12–25 (2018).
Prado, S. et al. Phenomics allows identification of genomic regions affecting maize stomatal conductance with conditional effects of water deficit and evaporative demand. Plant. Cell Environ. 41, 314–326 (2018).
Cooper, M. et al. Predicting the future of plant breeding: complementing empirical evaluation with genetic prediction. Crop Pasture Sci. 65, 311–336 (2014).
Ly, D. et al. Whole-genome prediction of reaction norms to environmental stress in bread wheat (Triticum aestivum L.) by genomic random regression. Field Crop. Res. 216, 32–41 (2018).
Rincent, R. et al. Using crop growth model stress covariates and AMMI decomposition to better predict genotype-by-environment interactions. Theor. Appl. Genet. 132, 3399–3411 (2019).
Technow, F., Messina, C. D., Totir, L. R. & Cooper, M. Integrating crop growth models with whole genome prediction through approximate bayesian computation. PLoS ONE 10, e0130855 (2015).
Robert, P., Le Gouis, J., Rincent, R. & The BreedWheat Consortium. Combining crop growth modeling with trait-assisted prediction improved the prediction of genotype by environment interactions. Front. Plant. Sci. 11, 827 (2020).
Welcker, C. et al. Physiological adaptive traits are a potential allele reservoir for maize genetic progress under challenging conditions. Nat. Commun. 13, 3225 (2022).
Halilou, O., Hamidou, F., Taya, B. K., Mahamane, S. & Vadez, V. Water use, transpiration efficiency and yield in cowpea (Vigna unguiculata) and peanut (Arachis hypogaea) across water regimes. Crop. Pasture Sci. 66, 715–728 (2015).
Holzworth, D. P. et al. Evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 62, 327–350 (2014).
Veyradier M., Christopher J. & Chenu K. in Proc. 7th Int. Conf. Functional-Structural Plant Models (eds Sievänen, R., Nikinmaa, E., Godin, C., Lintunen, A. & Nygren P.) 317–319 (2013).
Acknowledgements
V.V. was supported by the Make Our Planet Great Again (MOPGA) ICARUS project (Improve Crops in Arid Regions and future climates) funded by the Agence Nationale de la Recherche (ANR) (grant ANR-17-MPGA-0011), by the Occitanie Region through a financial contribution to grant ANR-17-MPGA-0011 and by Montpellier University of Excellence (I-Site MUSE). A.G. acknowledges support from the Agence National de la Recherche (PlastiMil grant ANR-20-CE20-0016). A.H. acknowledges support from the Bill and Melinda Gates Foundation projects ‘Stress tolerant rice for Africa and South Asia’ and ‘PlantDirect - Dry Direct Seeded Rice for the Indo-Gangetic Plains of India’. K.C. acknowledges support from the Australian Research Council (ARC Linkage Project LP210200723) and The University of Queensland. L.L. acknowledges support from the Agence National de la Recherche (SorDrought grant ANR-23-CE20-0052).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Earth & Environment thanks Cara Griffiths, Enli Wang, who co-reviewed with Die He, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vadez, V., Grondin, A., Chenu, K. et al. Crop traits and production under drought. Nat Rev Earth Environ 5, 211–225 (2024). https://doi.org/10.1038/s43017-023-00514-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43017-023-00514-w