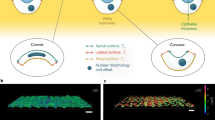
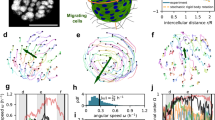
Abstract
Among the physicochemical cues in the cellular microenvironment that orchestrate cell processes, the different levels of curvature in the extracellular matrix and intrinsic to the tissues play a pivotal role in the spatiotemporal control of key cellular functions. Curvature influences multicellular organization and contributes to the onset of specific human diseases. This Review outlines how physical parameters used to describe the balance of forces in cells and tissues shed light on the mechanism of curvature sensing of cells across different length scales. We provide a summary of progress in delineating the fundamental mechanobiological characteristics of curvature sensing across various scales, emphasizing key challenges in the field. Additionally, we explore the potential of vertex model approaches to uncover critical physical elements involved in the mechanical regulation of curved tissues and the construction of functional architectures at the collective level. Finally, we examine how changes in curvature can influence transcriptional regulation through a reorganization of cytoskeletal forces acting on the nucleus, thereby facilitating the development of specific human diseases.
Key points
-
Cells interact with the curved surfaces of many organs and, at a lower scale, with the rounded features of the extracellular matrix, which inherently links geometric form and biological function.
-
The curvature of the extracellular matrix influences vital cellular processes by defining physical boundary conditions across multiple scales.
-
Multicellular tissues are active materials, and curved patterns in tissues emerge from stress fields generated by intrinsic forces coupled with extrinsic constraints.
-
The regulation of cellular shape and tension by curvatures at various length scales activates specific mechanotransduction pathways that synergistically determine downstream cellular functions.
-
Changes in the balance of cellular forces required to adapt to curved environments can have dramatic consequences, promoting the emergence of various human diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Procès, A., Luciano, M., Kalukula, Y., Ris, L. & Gabriele, S. Multiscale mechanobiology in brain physiology and diseases. Front. Cell Dev. Biol. 10, 823857 (2022).
Lantoine, J. et al. Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials 89, 14–24 (2016).
Riaz, M., Versaevel, M., Mohammed, D., Glinel, K. & Gabriele, S. Persistence of fan-shaped keratocytes is a matrix-rigidity-dependent mechanism that requires α5β1 integrin engagement. Sci. Rep. 6, 34141 (2016).
Saraswathibhatla, A., Indana, D. & Chaudhuri, O. Cell–extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24, 495–516 (2023).
Paul, C. D., Mistriotis, P. & Konstantopoulos, K. Cancer cell motility: lessons from migration in confined spaces. Nat. Rev. Cancer 17, 131–140 (2017).
Mohammed, D. et al. Substrate area confinement is a key determinant of cell velocity in collective migration. Nat. Phys. 15, 858–866 (2019).
Lomakin, A. J. et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894 (2020).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Adu-Berchie, K. et al. Generation of functionally distinct T-cell populations by altering the viscoelasticity of their extracellular matrix. Nat. Biomed. Eng. 7, 1374–1391 (2023).
Elosegui-Artola, A. et al. Matrix viscoelasticity controls spatiotemporal tissue organization. Nat. Mater. 22, 117–127 (2023).
Trepat, X. et al. Universal physical responses to stretch in the living cell. Nature 447, 592–595 (2007).
Bruyère, C. et al. Actomyosin contractility scales with myoblast elongation and enhances differentiation through YAP nuclear export. Sci. Rep. 9, 15565 (2019).
Martino, F., Perestrelo, A. R., Vinarský, V., Pagliari, S. & Forte, G. Cellular mechanotransduction: from tension to function. Front. Physiol. 9, 824 (2018).
Lantoine, J. et al. Inflammatory molecules released by mechanically injured astrocytes trigger presynaptic loss in cortical neuronal networks. ACS Chem. Neurosci. 12, 3885–3897 (2021).
Fleszar, A. J., Walker, A., Kreeger, P. K. & Notbohm, J. Substrate curvature induces fallopian tube epithelial cell invasion via cell–cell tension in a model of ovarian cortical inclusion cysts. Integr. Biol. 11, 342–352 (2019).
Maechler, F. A., Allier, C., Roux, A. & Tomba, C. Curvature-dependent constraints drive remodeling of epithelia. J. Cell Sci. 132, jcs222372 (2019).
Mandrycky, C., Hadland, B. & Zheng, Y. 3D curvature-instructed endothelial flow response and tissue vascularization. Sci. Adv. 6, eabb3629 (2020).
Werner, M. et al. Surface curvature differentially regulates stem cell migration and differentiation via altered attachment morphology and nuclear deformation. Adv. Sci. 4, 1600347 (2017).
Messal, H. A. et al. Tissue curvature and apicobasal mechanical tension imbalance instruct cancer morphogenesis. Nature 566, 126–130 (2019).
Luciano, M. et al. Appreciating the role of cell shape changes in the mechanobiology of epithelial tissues. Biophys. Rev. 3, 011305 (2022).
Mohammed, D. et al. Innovative tools for mechanobiology: unravelling outside-in and inside-out mechanotransduction. Front. Bioeng. Biotechnol. 7, 162 (2019).
Basu, R., Munteanu, E. L. & Chang, F. Role of turgor pressure in endocytosis in fission yeast. Mol. Biol. Cell 25, 549–727 (2014).
Roffay, C. et al. Passive coupling of membrane tension and cell volume during active response of cells to osmosis. Proc. Natl Acad. Sci. USA 118, e2103228118 (2021).
Latorre, E. et al. Active superelasticity in three-dimensional epithelia of controlled shape. Nature 563, 203–208 (2018).
Jentsch, T. J., Lutter, D., Planells-Cases, R., Ullrich, F. & Voss, F. K. VRAC: molecular identification as LRRC8 heteromers with differential functions. Pflug. Arch. Eur. J. Physiol. 468, 385–393 (2016).
Houdusse, A. & Sweeney, H. L. How myosin generates force on actin filaments. Trends Biochem. Sci. 41, 989–997 (2016).
Hill, T. L. & Kirschner, M. W. Bioenergetics and kinetics of microtubule and actin filament assembly–disassembly. Int. Rev. Cytol. 78, 1–125 (1982).
Molodtsov, M. I., Grishchuk, E. L., Efremov, A. K., McIntosh, J. R. & Ataullakhanov, F. I. Force production by depolymerizing microtubules: a theoretical study. Proc. Natl Acad. Sci. USA 102, 4353–4358 (2005).
Matis, M. The mechanical role of microtubules in tissue remodeling. BioEssays 42, 1900244 (2020).
Kozlov, M. M. & Chernomordik, L. V. Membrane tension and membrane fusion. Curr. Opin. Struct. Biol. 33, 61–67 (2015).
Gibbs, J. W. The Scientific Papers of J. Willard Gibbs (Dover Publications, Inc., 1961).
De Belly, H. et al. Actin-driven protrusions generate rapid long-range membrane tension propagation in cells. Preprint at bioRxiv https://doi.org/10.1101/2022.09.07.507005 (2022).
Lieber, A. D., Yehudai-Resheff, S., Barnhart, E. L., Theriot, J. A. & Keren, K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol. 23, 1409–1417 (2013).
Mueller, J. et al. Load adaptation of lamellipodial actin networks. Cell 171, 188–200.e16 (2017).
Hetmanski, J. H. R. et al. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 51, 460–475.e10 (2019).
Taubenberger, A. V., Baum, B. & Matthews, H. K. The mechanics of mitotic cell rounding. Front. Cell Dev. Biol. 8, 687 (2020).
Sedzinski, J. et al. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 476, 462–466 (2011).
Bertet, C., Sulak, L. & Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004).
Kruse, K., Joanny, J. F., Jülicher, F., Prost, J. & Sekimoto, K. Generic theory of active polar gels: a paradigm for cytoskeletal dynamics. Eur. Phys. J. E 16, 5–16 (2005).
Berezney, J., Goode, B. L., Fraden, S. & Dogic, Z. Extensile to contractile transition in active microtubule–actin composites generates layered asters with programmable lifetimes. Proc. Natl Acad. Sci. USA 119, e2115895119 (2022).
Saw, T. B. et al. Topological defects in epithelia govern cell death and extrusion. Nature 544, 212–216 (2017).
Blanch-Mercader, C., Guillamat, P., Roux, A. & Kruse, K. Quantifying material properties of cell monolayers by analyzing integer topological defects. Phys. Rev. Lett. 126, 028101 (2021).
Blanch-Mercader, C., Guillamat, P., Roux, A. & Kruse, K. Integer topological defects of cell monolayers: mechanics and flows. Phys. Rev. E 103, 012405 (2021).
Balasubramaniam, L. et al. Investigating the nature of active forces in tissues reveals how contractile cells can form extensile monolayers. Nat. Mater. 20, 1156–1166 (2021).
Dumortier, J. G. et al. Hydraulic fracturing and active coarsening position the lumen of the mouse blastocyst. Science 365, 465–468 (2019).
Kosmalska, A. J. et al. Physical principles of membrane remodelling during cell mechanoadaptation. Nat. Commun. 6, 7292 (2015).
Dietrich, J.-E. & Hiiragi, T. Stochastic patterning in the mouse pre-implantation embryo. Development 134, 4219–4231 (2007).
Rhumbler, L. Zur Mechanik des Gastrulationsvorganges insbesondere der Invagination: Eine entwickelungsmechanische Studie. Arch. Für. Entwickl. Org. 14, 401–476 (1902).
Moore, A. R. & Burt, A. S. On the locus and nature of the forces causing gastrulation in the embryos of Dendraster excentricus. J. Exp. Zool. 82, 159–171 (1939).
Martin, A. C. & Goldstein, B. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141, 1987–1998 (2014).
Gilmour, D., Rembold, M. & Leptin, M. From morphogen to morphogenesis and back. Nature 541, 311–320 (2017).
Pérez-González, C. et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat. Cell Biol. 23, 745–757 (2021).
Yang, Q. et al. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat. Cell Biol. 23, 733–744 (2021).
Haas, P. A. & Goldstein, R. E. Morphoelasticity of large bending deformations of cell sheets during development. Phys. Rev. E 103, 022411 (2021).
Höhn, S., Honerkamp-Smith, A. R., Haas, P. A., Trong, P. K. & Goldstein, R. E. Dynamics of a Volvox embryo turning itself inside out. Phys. Rev. Lett. 114, 178101 (2015).
Collinet, C. & Lecuit, T. Programmed and self-organized flow of information during morphogenesis. Nat. Rev. Mol. Cell Biol. 22, 245–265 (2021).
Münster, S. et al. Attachment of the blastoderm to the vitelline envelope affects gastrulation of insects. Nature 568, 395–399 (2019).
Merzouki, A., Malaspinas, O., Trushko, A., Roux, A. & Chopard, B. Influence of cell mechanics and proliferation on the buckling of simulated tissues using a vertex model. Nat. Comput. 17, 511–519 (2018).
Rauzi, M., Hočevar Brezavšček, A., Ziherl, P. & Leptin, M. Physical models of mesoderm invagination in Drosophila embryo. Biophys. J. 105, 3–10 (2013).
Hannezo, E., Prost, J. & Joanny, J.-F. Instabilities of monolayered epithelia: shape and structure of villi and crypts. Phys. Rev. Lett. 107, 078104 (2011).
Trushko, A. et al. Buckling of an epithelium growing under spherical confinement. Dev. Cell 54, 655–668.e6 (2020).
Tozluoǧlu, M. et al. Planar differential growth rates initiate precise fold positions in complex epithelia. Dev. Cell 51, 299–312.e4 (2019).
Hughes, A. J. et al. Engineered tissue folding by mechanical compaction of the mesenchyme. Dev. Cell 44, 165–178 (2018).
Jain, S. et al. The role of single-cell mechanical behaviour and polarity in driving collective cell migration. Nat. Phys. 16, 802–809 (2020).
Guillamat, P., Blanch-Mercader, C., Pernollet, G., Kruse, K. & Roux, A. Integer topological defects organize stresses driving tissue morphogenesis. Nat. Mater. 21, 588–597 (2022).
Bell, S., Lin, S.-Z., Rupprecht, J.-F. & Prost, J. Active nematic flows over curved surfaces. Phys. Rev. Lett. 129, 118001 (2022).
Fouchard, J. et al. Curling of epithelial monolayers reveals coupling between active bending and tissue tension. Proc. Natl Acad. Sci. USA 117, 9377–9383 (2020).
Blonski, S. et al. Direction of epithelial folding defines impact of mechanical forces on epithelial state. Dev. Cell 56, 3222–3234.e6 (2021).
Tomba, C., Luchnikov, V., Barberi, L., Blanch-Mercader, C. & Roux, A. Epithelial cells adapt to curvature induction via transient active osmotic swelling. Dev. Cell 57, 1257–1270.e5 (2022).
Gómez-González, M., Latorre, E., Arroyo, M. & Trepat, X. Measuring mechanical stress in living tissues. Nat. Rev. Phys. 2, 300–317 (2020).
Mehlenbacher, R. D., Kolbl, R., Lay, A. & Dionne, J. A. Nanomaterials for in vivo imaging of mechanical forces and electrical fields. Nat. Rev. Mater. 3, 17080 (2018).
Tambe, D. T. et al. Monolayer stress microscopy: limitations, artifacts, and accuracy of recovered intercellular stresses. PLoS ONE 8, e55172 (2013).
Trepat, X. et al. Physical forces during collective cell migration. Nat. Phys. 5, 426–430 (2009).
Ng, M. R., Besser, A., Brugge, J. S. & Danuser, G. Mapping the dynamics of force transduction at cell–cell junctions of epithelial clusters. eLife 3, e03282 (2014).
Vercruysse, E. et al. Geometry-driven migration efficiency of autonomous epithelial cell clusters. Preprint at bioRxiv https://doi.org/10.1101/2022.07.17.500364 (2022).
Marín-Llauradó, A. et al. Mapping mechanical stress in curved epithelia of designed size and shape. Nat. Commun. 14, 4014 (2023).
Mughal, A., Cox, S. J., Weaire, D., Burke, S. R. & Hutzler, S. Demonstration and interpretation of ‘scutoid’ cells formed in a quasi-2D soap froth. Philos. Mag. Lett. 98, 358–364 (2018).
Gómez-Gálvez, P., Vicente-Munuera, P., Anbari, S., Buceta, J. & Escudero, L. M. The complex three-dimensional organization of epithelial tissues. Development 148, dev195669 (2021).
Gómez-Gálvez, P. et al. Scutoids are a geometrical solution to three-dimensional packing of epithelia. Nat. Commun. 9, 2960 (2018).
Gómez, H. F., Dumond, M. S., Hodel, L., Vetter, R. & Iber, D. 3D cell neighbour dynamics in growing pseudostratified epithelia. eLife 10, e68135 (2021).
Rupprecht, J.-F. et al. Geometric constraints alter cell arrangements within curved epithelial tissues. MBoC 28, 3582–3594 (2017).
Lou, Y., Rupprecht, J.-F., Theis, S., Hiraiwa, T. & Saunders, T. E. Curvature-induced cell rearrangements in biological tissues. Phys. Rev. Lett. 130, 108401 (2023).
Hannezo, E., Prost, J. & Joanny, J.-F. Theory of epithelial sheet morphology in three dimensions. Proc. Natl Acad. Sci. USA 111, 27–32 (2014).
Luciano, M. et al. Cell monolayers sense curvature by exploiting active mechanics and nuclear mechanoadaptation. Nat. Phys. 17, 1382–1390 (2021).
Odell, G. M., Oster, G., Alberch, P. & Burnside, B. The mechanical basis of morphogenesis. Dev. Biol. 85, 446–462 (1981).
Fletcher, A. G., Osterfield, M., Baker, R. E. & Shvartsman, S. Y. Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291–2304 (2014).
Honda, H. & Eguchi, G. How much does the cell boundary contract in a monolayered cell sheet? J. Theor. Biol. 84, 575–588 (1980).
Nagai, T. & Honda, H. A dynamic cell model for the formation of epithelial tissues. Philos. Mag. Pt B 81, 699–719 (2001).
Farhadifar, R., Röper, J.-C., Aigouy, B., Eaton, S. & Jülicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Hočevar Brezavšček, A., Rauzi, M., Leptin, M. & Ziherl, P. A model of epithelial invagination driven by collective mechanics of identical cells. Biophys. J. 103, 1069–1077 (2012).
Sumi, A. et al. Adherens junction length during tissue contraction is controlled by the mechanosensitive activity of actomyosin and junctional recycling. Dev. Cell 47, 453–463.e3 (2018).
Théry, M., Pépin, A., Dressaire, E., Chen, Y. & Bornens, M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil. Cytoskelet. 63, 341–355 (2006).
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M. & Ingber, D. E. Geometric control of cell life and death. Science 276, 1425 (1997).
Thery, M. et al. Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl Acad. Sci. USA 103, 19771–19776 (2006).
Parker, K. K. et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 16, 1195–1204 (2002).
Versaevel, M., Grevesse, T. & Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 3, 671 (2012).
Senger, F. et al. Spatial integration of mechanical forces by α-actinin establishes actin network symmetry. J. Cell Sci. 132, jcs236604 (2019).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Bischofs, I. B., Klein, F., Lehnert, D., Bastmeyer, M. & Schwarz, U. S. Filamentous network mechanics and active contractility determine cell and tissue shape. Biophys. J. 95, 3488–3496 (2008).
Schakenraad, K. et al. Mechanical interplay between cell shape and actin cytoskeleton organization. Soft Matter 16, 6328–6343 (2020).
James, J., Goluch, E. D., Hu, H., Liu, C. & Mrksich, M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell Motil. Cytoskelet. 65, 841–852 (2008).
Vignaud, T., Blanchoin, L. & Théry, M. Directed cytoskeleton self-organization. Trends Cell Biol. 22, 671–682 (2012).
Ladoux, B., Mège, R.-M. & Trepat, X. Front–rear polarization by mechanical cues: from single cells to tissues. Trends Cell Biol. 26, 420–433 (2016).
Lam, N. T., Muldoon, T. J., Quinn, K. P., Rajaram, N. & Balachandran, K. Valve interstitial cell contractile strength and metabolic state are dependent on its shape. Integr. Biol. 8, 1079–1089 (2016).
Gupta, S. K., Li, Y. & Guo, M. Anisotropic mechanics and dynamics of a living mammalian cytoplasm. Soft Matter 15, 190–199 (2019).
Chen, T. et al. Large-scale curvature sensing by directional actin flow drives cellular migration mode switching. Nat. Phys. 15, 393–402 (2019).
Anon, E. et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl Acad. Sci. USA 109, 10891–10896 (2012).
Begnaud, S., Chen, T., Delacour, D., Mège, R.-M. & Ladoux, B. Mechanics of epithelial tissues during gap closure. Curr. Opin. Cell Biol. 42, 52–62 (2016).
Ravasio, A. et al. Gap geometry dictates epithelial closure efficiency. Nat. Commun. 6, 7683 (2015).
Sandu, G. et al. Kinked silicon nanowires: superstructures by metal-assisted chemical etching. Nano Lett. 19, 7681–7690 (2019).
Huang, J. et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 9, 1111–1116 (2009).
Arnold, M. et al. Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 5, 383–388 (2004).
Oria, R. et al. Force loading explains spatial sensing of ligands by cells. Nature 552, 219–224 (2017).
Schaumann, E. N. & Tian, B. Actin-packed topography: cytoskeletal response to curvature. Proc. Natl Acad. Sci. USA 116, 22897–22898 (2019).
Carey, S. P. et al. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. 8, 821–835 (2016).
Kennedy, K. M., Bhaw-Luximon, A. & Jhurry, D. Cell–matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: implications for scaffold design and performance. Acta Biomater. 50, 41–55 (2017).
Koons, B. et al. Cancer protrusions on a tightrope: nanofiber curvature contrast quantitates single protrusion dynamics. ACS Nano 11, 12037–12048 (2017).
Mukherjee, A., Behkam, B. & Nain, A. S. Cancer cells sense fibers by coiling on them in a curvature-dependent manner. iScience 19, 905–915 (2019).
Zhang, W. et al. Curved adhesions mediate cell attachment to soft matrix fibres in three dimensions. Nat. Cell Biol. 25, 1453–1464 (2023).
Weiss, P. Experiments on cell and axon orientation in vitro: the role of colloidal exudates in tissue organization. J. Exp. Zool. 100, 353–386 (1945).
Dunn, G. A. & Heath, J. P. A new hypothesis of contact guidance in tissue cells. Exp. Cell Res. 101, 1–14 (1976).
Bade, N. D., Kamien, R. D., Assoian, R. K. & Stebe, K. J. Curvature and Rho activation differentially control the alignment of cells and stress fibers. Sci. Adv. 3, e1700150 (2017).
Iruela-Arispe, M. L. & Davis, G. E. Cellular and molecular mechanisms of vascular lumen formation. Dev. Cell 16, 222–231 (2009).
Sims, D. E. The pericyte — a review. Tissue Cell 18, 153–174 (1986).
O’Connor, C., Brady, E., Zheng, Y., Moore, E. & Stevens, K. R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 7, 702–716 (2022).
Yu, S.-M. Substrate curvature affects the shape, orientation, and polarization of renal epithelial cells. Acta Biomater. 77, 311–321 (2018).
Biton, Y. Y. & Safran, S. A. The cellular response to curvature-induced stress. Phys. Biol. 6, 046010 (2009).
Bade, N. D., Xu, T., Kamien, R. D., Assoian, R. K. & Stebe, K. J. Gaussian curvature directs stress fiber orientation and cell migration. Biophys. J. 114, 1467–1476 (2018).
Kemkemer, R., Teichgräber, V., Schrank-Kaufmann, S., Kaufmann, D. & Gruler, H. Nematic order–disorder state transition in a liquid crystal analogue formed by oriented and migrating amoeboid cells. Eur. Phys. J. E 3, 101–110 (2000).
Duclos, G., Garcia, S., Yevick, H. G. & Silberzan, P. Perfect nematic order in confined monolayers of spindle-shaped cells. Soft Matter 10, 2346–2353 (2014).
Harmand, N., Huang, A. & Hénon, S. 3D shape of epithelial cells on curved substrates. Phys. Rev. X 11, 031028 (2021).
Harmand, N., Dervaux, J., Poulard, C. & Hénon, S. Thickness of epithelia on wavy substrates: measurements and continuous models. Eur. Phys. J. E 45, 53 (2022).
Werner, M., Kurniawan, N. A., Korus, G., Bouten, C. V. C. & Petersen, A. Mesoscale substrate curvature overrules nanoscale contact guidance to direct bone marrow stromal cell migration. J. R. Soc. Interface 15, 20180162 (2018).
Asano, N., Sugihara, S., Suye, S. & Fujita, S. Electrospun porous nanofibers with imprinted patterns induced by phase separation of immiscible polymer blends. ACS Omega 7, 19997–20005 (2022).
Kumbar, S. G., Nukavarapu, S. P., James, R., Nair, L. S. & Laurencin, C. T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 29, 4100–4107 (2008).
Bowers, D. T. & Brown, J. L. Nanofiber curvature with Rho GTPase activity increases mouse embryonic fibroblast random migration velocity. Integr. Biol. 13, 295–308 (2021).
Qu, J. et al. Optimization of electrospun TSF nanofiber alignment and diameter to promote growth and migration of mesenchymal stem cells. Appl. Surf. Sci. 261, 320–326 (2012).
DiMilla, P., Stone, J., Quinn, J., Albelda, S. & Lauffenburger, D. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J. Cell Biol. 122, 729–737 (1993).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Schreiber, C., Amiri, B., Heyn, J. C. J., Rädler, J. O. & Falcke, M. On the adhesion–velocity relation and length adaptation of motile cells on stepped fibronectin lanes. Proc. Natl Acad. Sci. USA 118, e2009959118 (2021).
Badami, A. S., Kreke, M. R., Thompson, M. S., Riffle, J. S. & Goldstein, A. S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 27, 596–606 (2006).
Tian, F. et al. Quantitative analysis of cell adhesion on aligned micro‐ and nanofibers. J. Biomed. Mater. Res. 84A, 291–299 (2008).
Noriega, S. E., Hasanova, G. I., Schneider, M. J., Larsen, G. F. & Subramanian, A. Effect of fiber diameter on the spreading, proliferation and differentiation of chondrocytes on electrospun chitosan matrices. Cell Tissues Organs 195, 207–221 (2012).
Pieuchot, L. et al. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat. Commun. 9, 3995 (2018).
Werner, M., Petersen, A., Kurniawan, N. A. & Bouten, C. V. C. Cell-perceived substrate curvature dynamically coordinates the direction, speed, and persistence of stromal cell migration. Adv. Biosys. 3, 1900080 (2019).
He, X. & Jiang, Y. Substrate curvature regulates cell migration. Phys. Biol. 14, 035006 (2017).
Hegarty-Cremer, S. G. D., Simpson, M. J., Andersen, T. L. & Buenzli, P. R. Modelling cell guidance and curvature control in evolving biological tissues. J. Theor. Biol. 520, 110658 (2021).
Yevick, H. G., Duclos, G., Bonnet, I. & Silberzan, P. Architecture and migration of an epithelium on a cylindrical wire. Proc. Natl Acad. Sci. USA 112, 5944–5949 (2015).
Xi, W., Sonam, S., Lim, C. T. & Ladoux, B. Tubular microscaffolds for studying collective cell migration. in Methods in Cell Biology Vol. 146, 3–21 (Elsevier, 2018).
Ye, M. et al. Brain microvascular endothelial cells resist elongation due to curvature and shear stress. Sci. Rep. 4, 4681 (2014).
Rougerie, P. et al. Topographical curvature is sufficient to control epithelium elongation. Sci. Rep. 10, 14784 (2020).
Bidan, C. M. et al. How linear tension converts to curvature: geometric control of bone tissue growth. PLoS ONE 7, e36336 (2012).
Ehrig, S. et al. Surface tension determines tissue shape and growth kinetics. Sci. Adv. 5, 7 (2019).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 10, 469–475 (2011).
Glentis, A. et al. The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces. Sci. Adv. 8, eabn5406 (2022).
Brandstätter, T. Curvature induces active velocity waves in rotating spherical tissues. Nat. Commun. 14, (2023).
Luciano, M., Versaevel, M., Kalukula, Y. & Gabriele, S. Mechanoresponse of curved epithelial monolayers lining bowl‐shaped 3D microwells. Adv. Healthc. Mater. 13, 2203377 (2024).
Shellard, A. & Mayor, R. Durotaxis: the hard path from in vitro to in vivo. Dev. Cell 56, 227–239 (2021).
Christopherson, G. T., Song, H. & Mao, H.-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 30, 556–564 (2009).
Di Meglio, I. et al. Pressure and curvature control of the cell cycle in epithelia growing under spherical confinement. Cell Rep. 40, 111227 (2022).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017).
Kalukula, Y., Stephens, A. D., Lammerding, J. & Gabriele, S. Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 23, 583–602 (2022).
Gjorevski, N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021 (2022).
Andreu, I. Mechanical force application to the nucleus regulates nucleocytoplasmic transport. Nat. Cell Biol. 24, 896–905 (2022).
Ruiz, S. A. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cell 26, 2921–2927 (2008).
Yang, Y. et al. Gaussian curvature-driven direction of cell fate toward osteogenesis with triply periodic minimal surface scaffolds. Proc. Natl Acad. Sci. USA 119, e2206684119 (2022).
Van der Putten, C., van den Broek, D. & Kurniawan, N. A. Myofibroblast transdifferentiation of keratocytes results in slower migration and lower sensitivity to mesoscale curvatures. Front. Cell Dev. Biol. 10, 930373 (2022).
Connon, C. J. & Gouveia, R. M. Milliscale substrate curvature promotes myoblast self‐organization and differentiation. Adv. Biol. 5, e2000280 (2021).
Xu, X. et al. Histone modification of osteogenesis related genes triggered by substrate topography promotes human mesenchymal stem cell differentiation. ACS Appl. Mater. Interfaces 15, 29752–29766 (2023).
Lee, J., Nishikawa, R. M., Reiser, I., Boone, J. M. & Lindfors, K. K. Local curvature analysis for classifying breast tumors: preliminary analysis in dedicated breast CT: local curvature analysis for classifying breast tumors. Med. Phys. 42, 5479–5489 (2015).
Tan, S. et al. Adaptive region-growing with maximum curvature strategy for tumor segmentation in 18 F-FDG PET. Phys. Med. Biol. 62, 5383–5402 (2017).
Arvanitis, C., Khuon, S., Spann, R., Ridge, K. M. & Chew, T.-L. Structure and biomechanics of the endothelial transcellular circumferential invasion array in tumor invasion. PLoS ONE 9, e89758 (2014).
Zhu, P. et al. Biomechanical microenvironment regulates fusogenicity of breast cancer cells. ACS Biomater. Sci. Eng. 5, 3817–3827 (2019).
Wullkopf, L. et al. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 29, 2378–2385 (2018).
Nemec, S. et al. Interfacial curvature in confined coculture directs stromal cell activity with spatial corralling of pancreatic cancer cells. Adv. Biol. 5, 2000525 (2021).
Lee, J., Abdeen, A. A., Wycislo, K. L., Fan, T. M. & Kilian, K. A. Interfacial geometry dictates cancer cell tumorigenicity. Nat. Mater. 15, 856–862 (2016).
Franklin, J. M. Insights into recent findings and clinical application of YAP and TAZ in cancer. Nat. Rev. Cancer 23, 512–525 (2023).
Fierling, J. et al. Embryo-scale epithelial buckling forms a propagating furrow that initiates gastrulation. Nat. Commun. 13, 3348 (2022).
Villedieu, A. et al. Homeotic compartment curvature and tension control spatiotemporal folding dynamics. Nat. Commun. 14, 594 (2023).
Wang, T., Dai, Z., Potier-Ferry, M. & Xu, F. Curvature-regulated multiphase patterns in tori. Phys. Rev. Lett. 130, 048201 (2023).
Hirashima, T. & Matsuda, M. ERK-mediated curvature feedback regulates branch. Morphogene. Lung Epithel. Tissue https://doi.org/10.1101/2021.07.11.451982 (2021).
Huang, C.-K., Yong, X., She, D. T. & Teck, C. Surface curvature and basal hydraulic stress induce spatial bias in cell extrusion. https://doi.org/10.1101/2022.04.01.486717 (2022).
Roy, A. et al. Programmable tissue folding patterns in structured hydrogels. Adv. Mater. https://doi.org/10.1002/adma.202300017 (2023).
Kalukula, Y., Luciano, M. & Gabriele, S. Translating cell mechanobiology and nuclear deformations to the clinic. Clin. Trans. Med. 12, e1000 (2022).
Doostmohammadi, A. & Ladoux, B. Physics of liquid crystals in cell biology. Trends Cell Biol. 32, 140–150 (2022).
Lou, H.-Y. et al. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl Acad. Sci. USA 116, 23143–23151 (2019).
Wagstyl, K. et al. BigBrain 3D atlas of cortical layers: cortical and laminar thickness gradients diverge in sensory and motor cortices. PLoS Biol. 18, e3000678 (2020).
Conrad, L. et al. The biomechanical basis of biased epithelial tube elongation in lung and kidney development. Development 148, dev194209 (2021).
Mederacke, M., Conrad, L., Vetter, R. & Iber, D. Geometric effects position renal vesicles during kidney development. Cell Rep. https://doi.org/10.1101/2022.08.30.505859 (2022).
Peurla, M. et al. Morphometric analysis of the terminal ductal lobular unit architecture in human breast. Preprint at bioRxiv https://doi.org/10.1101/2023.03.12.532249v1.full.pdf (2023).
Sung, J. H., Yu, J., Luo, D., Shuler, M. L. & March, J. C. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab. Chip 11, 389–392 (2011).
Silver, F. H., Freeman, J. W. & Seehra, G. P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 36, 1529–1553 (2003).
Wershof, E. et al. Matrix feedback enables diverse higher-order patterning of the extracellular matrix. PLoS Comput. Biol. 15, e1007251 (2019).
Park, D. et al. Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nat. Mater. 19, 227–238 (2020).
Reznikov, N., Shahar, R. & Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 10, 3815–3826 (2014).
Sartori, P., Geyer, V. F., Howard, J. & Jülicher, F. Curvature regulation of the ciliary beat through axonemal twist. Phys. Rev. E 94, 042426 (2016).
Chabanon, M. & Rangamani, P. Geometric coupling of helicoidal ramps and curvature-inducing proteins in organelle membranes. J. R. Soc. Interface 16, 20190354 (2019).
Hu, J. et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319, 1247–1250 (2008).
Collado, J. et al. Tricalbin-mediated contact sites control ER curvature to maintain plasma membrane integrity. Dev. Cell 51, 476–487.e7 (2019).
Tozluoǧlu, M. & Mao, Y. On folding morphogenesis, a mechanical problem. Philos. Trans. R. Soc. B 375, 20190564 (2020).
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499 (2009).
Storgel, N., Krajnc, M., Mrak, P., Štrus, J. & Ziherl, P. Quantitative morphology of epithelial folds. Biophys. J. 110, 269–277 (2016).
Sui, L. et al. Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms. Nat. Commun. 9, 4620 (2018).
Wang, Y. et al. A microengineered collagen scaffold for generating a polarized crypt–villus architecture of human small intestinal epithelium. Biomaterials 128, 44–55 (2017).
Savin, T. et al. On the growth and form of the gut. Nature 476, 57–62 (2011).
Huycke, T. R. et al. Genetic and mechanical regulation of intestinal smooth muscle development. Cell 179, 90–105.e21 (2019).
Garcia, K. E., Kroenke, C. D. & Bayly, P. V. Mechanics of cortical folding: stress, growth and stability. Philos. Trans. R. Soc. B 373, 20170321 (2018).
Callens, S. J. P. & Zadpoor, A. A. From flat sheets to curved geometries: origami and kirigami approaches. Mater. Today 21, 241–264 (2018).
Acknowledgements
The authors apologize to all authors whose work could not be included owing to space constraints. S.G. acknowledges funding from FEDER Prostem Research Project no. 1510614 (Wallonia DG06), the F.R.S.-FNRS Epiforce Project no. T.0092.21, the F.R.S.-FNRS Cellsqueezer Project no. J.0061.23, the F.R.S.-FNRS Optopattern Project no. U.NO26.22, Programme Wallon d’Investissement Région Wallone pour les instruments d’imagerie (INSTIMAG UMONS #1910169) and the Interreg MAT(T)ISSE project, which is financially supported by Interreg France-Wallonie-Vlaanderen (Fonds Européen de Développement Régional, FEDER-ERDF). M.L. is financially supported by a WBI.World Scholarship Fellowship from the Wallonia-Brussels International (WBI) Excellence Grants Programme. A.R. acknowledges funding from the Swiss National Fund for Research Grants nos 31003A_149975, 31003A_173087 and 31003A_200793, and the European Research Council Synergy Grant no. 951324_R2-TENSION. The authors want to thank the NCCR Chemical Biology for constant support during this project. C.T. acknowledges support from the French National Research Agency, Grant no. ANR-22-CE13-0015-01 for the project ‘CurvEDyn’.
Author information
Authors and Affiliations
Contributions
M.L., C.T., A.R. and S.G. conceptualized the article. Figure designs were generated by all authors and further edited by M.L. and S.G. All authors contributed substantially to the writing, the discussion of the content and approved the final content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Physics thanks Krisopher Kilian, Tsuyoshi Hirashima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adherens junction
-
Component of the cell–cell junction in multicellular organisms in which cadherin receptors bridge neighbouring plasma membranes via their homophilic interactions. Adherens junction provides strong mechanical attachment between neighbouring cells through the linkage of their cytoplasmic face to the actin cytoskeleton.
- BAR-domain protein
-
Highly conserved protein dimerization domains that occur in many proteins involved in membrane dynamics and act as connecting links between actin dynamics and membrane rearrangements in all eukaryotes. BAR domains preferentially bind to curved membrane regions.
- Chromatin
-
Complex of genomic DNA with proteins called histones that forms chromosomes within the nucleus of eukaryotic cells.
- Durotaxis
-
Guidance of cell migration by rigidity gradients, which arise from differential structural properties of the extracellular matrix. Most cells migrate up rigidity gradients (that is, in the direction of greater stiffness) but some cell types have been reported to migrate down rigidity gradients (known as negative durotaxis).
- Epithelial tissues
-
Single-cell monolayers that separate tissues or cavities from their environment.
- Filopodia
-
Slender and finger-like projections that extend from the leading edge of migrating cells. They are filled with bundled actin filaments and are involved in cell migration, cell–cell communication and sensing the environment.
- Focal adhesions
-
Multiprotein site within cells that mechanically interact the extracellular matrix (cell outside) with the actin cytoskeleton (cell inside).
- Gene transcription
-
Process of copying a segment of DNA sequence into an RNA molecule. This process can be divided in three steps: initiation, elongation and termination.
- Histone
-
Family of positively charged proteins that associate with DNA and help condense it into chromatin.
- Hypotonic shocks
-
Refer to an environmental medium that has a lower concentration of solutes than the cytoplasm, inducing a flow of water into the cell and a sudden modification of its osmotic pressure.
- Lamellipodia
-
Thin projection that extends from the leading edge of a migrating cell and contains a quasi-2D actin mesh. These projections on the leading edge are involved in cell migration and exploration of the environment.
- Laplace law
-
The pressure of a bubble with fixed surface tension varies inversely with its radius of curvature.
- Manifold
-
A topological space M for which every point x has a neighbourhood homeomorphic to Euclidean space. In simple terms, it is a mathematical concept describing a space that appears flat similar to ordinary Euclidean space when you zoom in close enough but can have a more complicated overall shape such as a curved shaped.
- Mechanosensing
-
Molecular process through which cells or cellular components translate mechanical forces or deformations into biochemical signals.
- Mechanotransduction
-
Cellular responses to changes in the mechanical environment, including forces, deformations or mechanical properties.
- Membrane tension
-
Local characteristic of the membrane, delineating the stretching–compression elastic stresses at each point along the membrane surface. This involves examining an infinitesimal element of the membrane plane, isolated by an imaginary boundary. Mechanical tension is the force exerted on the unit length of this imaginary boundary by the surrounding membrane, acting tangentially to the membrane plane.
- Mesenchymal stem cells (MSCs)
-
Type of multipotent stromal cell that can differentiate into various cell types, such as osteoblasts (bone cells), chondrocytes (cartilage cells) and adipocytes (fat cells).
- Microvilli
-
Finger-like projections of ~1–2 µm in length that extend from the surface of epithelial cells lining, for instance, the small intestine, kidney tubules and the inner ear. Microvilli are filled with bundled actin filaments and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion and mechanotransduction.
- Molecular clutch model
-
Concept that describes the flexible transmission of forces generated by the flow of actin filaments to adhesion sites, allowing cells to exert a spatially and temporally regulated grip on the substrate. It has a crucial role in the mechanical connection between the actin flow and cell adhesion complexes during cell migration.
- Nematic order
-
Refers to a state of molecular alignment observed in cellular populations such as fibroblasts, akin to the alignment seen in liquid crystals. In this state, molecular entities exhibit a preferred directionality without long-range positional order.
- Osmotic pressure
-
Pressure caused by a difference in the amounts of solutes (or molecules) between solutions (or fluids) separated by a semipermeable membrane.
- Second harmonic generation microscopy
-
A second-order coherent process is employed, up-converting two lower energy photons precisely to twice the incident frequency (half the wavelength) of an excitation laser. This nonlinear imaging technique has proven effective in the specific and sensitive visualization of endogenous extracellular matrix components, such as collagen fibres, across diverse sample types.
- Signal transduction
-
Process by which cells receive, interpret and respond to extracellular forces and signals. This intricate communication system involves the transmission of molecular signals through a series of events, ultimately leading to a cellular response or change in behaviour.
- Tessellation
-
Repeating pattern that covers a 2D surface without overlaps or gaps using one or more geometric shapes, called tiles, with no overlaps and no gaps.
- Transcription
-
Process by which the information in a strand of DNA is copied into a new molecule of messenger RNA.
- Vertex models
-
A class of mathematical models that treat cells as individual objects, represented by polygons in two dimensions and polyhedra in three dimensions. Epithelial tissues are modelled as a connected mesh of these polygons or polyhedral elements, and mechanical forces are applied to the vertices of these geometric structures.
- Villus and crypt domains
-
Two distinct regions of the intestinal epithelium, which play important roles in nutrient uptake and tissue regeneration, respectively. The villus is a finger-like projection lined with absorptive enterocytes, secretory enteroendocrine cells and goblet cells. The crypt is a pocket-like invagination located at the base of the villus that houses the intestinal stem cell niche.
- Viscoelasticity
-
Rheological property of biological tissues and materials that present elastic properties and viscous properties, which allow for timescale-dependent deformation when subjected to mechanical stress.
- Yes-associated protein
-
Yes-associated protein, also known as YAP, is a mechanosensitive transcriptional co-activator protein that associates with several DNA-binding proteins to drive gene transcription. YAP activity is regulated by many kinase cascade pathways and proteins through phosphorylation. Phosphorylated YAP can be sequestered in the cytoplasm and then degraded by the ubiquitin–proteasome system, whereas unphosphorylated YAP translocates to the nucleus, where it performs a series of functions. YAP and its paralogue, transcriptional co-activator with PDZ-binding motif (TAZ), are the major downstream effectors of the Hippo pathway.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luciano, M., Tomba, C., Roux, A. et al. How multiscale curvature couples forces to cellular functions. Nat Rev Phys 6, 246–268 (2024). https://doi.org/10.1038/s42254-024-00700-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42254-024-00700-9