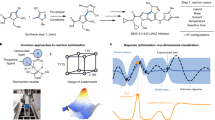
Abstract
Traditional optimization methods using one variable at a time approach waste time and chemicals and assume that different parameters are independent from one another. Hence, a simpler, more practical, and rapid process for predicting reaction conditions that can be applied to several manufacturing environmentally sustainable processes is highly desirable. In this study, biaryl compounds were synthesized efficiently using an organic Brønsted acid catalyst in a flow system. Bayesian optimization-assisted multi-parameter screening, which employs one-hot encoding and appropriate acquisition function, rapidly predicted the suitable conditions for the synthesis of 2-amino-2′-hydroxy-biaryls (maximum yield of 96%). The established protocol was also applied in an optimization process for the efficient synthesis of 2,2′-dihydroxy biaryls (up to 97% yield). The optimized reaction conditions were successfully applied to gram-scale synthesis. We believe our algorithm can be beneficial as it can screen a reactor design without complicated quantification and descriptors.
Similar content being viewed by others
Introduction
Data-driven methodology enables the rapid identification of appropriate conditions for eco-friendly and sustainable chemical processes1,2,3,4. Among these methods, computational and automated protocols identifying suitable reaction conditions in continuous-flow system have been extensively investigated owing to their reproducibility, rapid heating, mixing, short reaction periods, and ease of automation5,6,7,8,9,10,11,12,13,14. Furthermore, Gaussian process regression can efficiently predict the appropriate reaction parameters of a flow reaction by estimating yields from a small dataset15. Despite the significant advances in this field, it is still difficult to efficiently and simultaneously optimize multiple flow reaction variables (e.g., flow rate, pipe diameter, and length, micromixer (reactor) type, and also other conventional reaction parameters). In recent, Bayesian optimization (BO), which is a powerful probabilistic method of determining the global maximum (or minimum) of a black-box objective function, is useful for multi-parameter screening in flow platform as well as batch system16,17,18,19,20,21. Our group also applied the BO-assisted screening of numerical parameters for electrochemical oxidation of amines to ketimines and electrochemical reductive carboxylation in flow22,23.
To utilize categorical chemical variables (e.g., solvents and reagents) for data-driven reaction optimization, the steric and electronic properties of a molecule were converted to the corresponding numeric values with descriptors21,24,25,26, which required precise structural representation, the quantum chemical properties and theoretical calculations for the construction of a practical model. It is difficult for even if experienced chemists and scientists, to attain the chemical reaction’s dataset with the selected categorical parameters and minimum features. It is also challenging to convert dominant, non-numerical parameters into numerical parameters through the selection of proper physical and engineering features27,28,29,30,31,32, although these categorical parameters are crucial to achieve good outcomes. To demonstrate a simpler and more practical BO-assisted method of identifying optimal reaction conditions, we focused on the direct optimization of categorical parameters with neither feature extraction nor model construction. In this study, we enhanced the BO algorithm by adopting a categorical variable as an integer value via one-hot encoding without employing one-hot encoding to avoid the effect of a relative magnitude between integer values33,34 (e.g., mixer A: ‘0’ represented by 1 0 0, mixer B: ‘1’ represented by 0 1 0, mixer C: ‘2’ represented by 0 0 1). A categorical variable can be rounded to the closest integer and induced to the appropriate value, along with the optimization of a larger number of continuous numerical factors. Using BO-assisted parallel screening of six numerical and categorical parameters, appropriate continuous flow synthetic conditions were determined for the production of functionalized biaryls via the redox-neutral cross-coupling reaction of iminoquinone monoacetals (IQMAs) or quinone monoacetals (QMAs) with arenols (Fig. 1).
Functionalized biaryl motifs are found in numerous natural products35,36, pharmaceuticals37,38,39, and chiral ligands40,41,42,43. These biaryl compounds are typically synthesized by transition-metal-catalyzed reactions such as cross-coupling and oxidative coupling of aryl compounds in a regio- and stereoselective manner44,45,46,47,48,49,50,51,52. More recently, an efficient, metal-free, and operationally simple organocatalysis process has been developed for synthesis of arenol-derived biaryls. In 2016, two independent studies reported readily available organic Brønsted acid-catalyzed cross-coupling in batch reactions of QMAs or IQMAs with arenols to afford phenol biaryls with the following organic acids: TFA or (PhO)2PO2H51; MeSO3H52. Although both studies reported good yields and a wide substrate scope, the reaction conditions had drawbacks, such as the high catalyst loading (20 mol%), long reaction period (16 h), and high reaction temperature (at 100 °C). We envisaged a flow system with higher mixing efficiency, good thermal conductivity, and easy reaction control could address more effective and green-sustainable synthetic process for the organocatalyzed cross-coupling without a formation of by-product.
Results and discussion
BO-assisted parallel screening of flow reaction conditions and evaluation of substrate scope
To determine a practical optimization methodology for the flow reaction conditions, we used IQMA 1a, 2-naphthol 2a, and a catalytic amount of TfOH in toluene to conduct six reactions to screen five continuous numerical parameters and one categorical parameter: amount of 2a (1–3 equiv.), temperature (20–60 °C), concentration of 1a in toluene (0.01–0.1 M), flow rate (0.05–0.2 mL/min), catalyst loading (0.5–2 mol%), and mixer type (Comet X, β-type, and T-shaped; for more information about micromixers, see Fig. S2 in Supplementary Note 3). We set a broader initial dataset using six, rather than three datapoints which can be inadequate to find suitable conditions, avoid expensive solvents and toxic reagent, and decrease the amount of chemicals (see Supplementary Data 2). The results are summarized in Table 1, entries 1–6.
On the other hand, using TfOH in a batch system did not afford the desired biaryls 3 due to the fast decomposition of IQMAs 1 under the strong acidic conditions of TfOH, and the generation of various side products. In the flow system, the rapid mixing of substrates and quenching of TfOH improved the yield by suppressing these undesired reaction pathways.
In our study, we employed BO using parallel lower confidence bounds (LCB) as an acquisition function53. Parallel BO efficiently evaluates an expensive objective function at several points, simultaneously. Optimization of the mixers was not efficiently achieved using other acquisition functions such as single EI (expected improvement), LCB, and parallel EI. With a batch size of three, each mixer was suggested along with next numerical parameters to examine (entries 7–9) based on the initial dataset in entries 1–6. The evaluation of these three estimated conditions by experiments reported a slightly improved yield (up to 81%) under the reaction conditions corresponding to entry 7. Further consideration of entries 1–9 with the BO protocol suggested the β-type mixer was not appropriate for the coupling of 1a with 2a as shown in entries 10–12. However, no improvement in the yield was observed with a use of Comet X and T-shaped mixers (maximum yield of 79%). Finally, we acquired three different experimental datapoints (entries 13–15) with Comet X through BO-assisted optimization based on the 12 conditions in entries 1–12. Gratifyingly, the desired product 3a was obtained in 93% isolated yield using a microflow system (Comet X micro mixer, flow rate = 0.08 mL/min, and residence time = 15 min) as shown in entry 15. Screening of microreactors (T-shape, β-type, and Comet-X) showed Comet-X had superior performance under the same reaction conditions (see Scheme S1 in Supplementary Note 4).
Having BO-established suitable reaction conditions, we examined the substrate scope for the flow synthesis of biaryls using a variety of IQMAs 1 and arenols 2 in Fig. 2. IQMAs (1b–d) bearing an electron-donating or withdrawing substituent at the 4-position of the phenyl group; they were reacted with 2-naphthol (2a) to synthesize the corresponding products 3b–d with good yields (86–93%). The sterically less hindered or bulky sulfonyl groups also produced biaryls 3e and 3f in 92 and 81% yields, respectively. The product 3g was successfully obtained in 76% yield from methoxycarbonyl-protected IQMA 1g. Similarly, mono-substituted IQMAs and iminonaphthoquinone monoacetals 1h–j were converted into the corresponding coupling products 3h–j (77–96% yields). The enantioselective coupling reaction of IQMA 1h and 2-naphthol 2a was tested using chiral phosphoric acid and the product 3h was obtained in 78% yield with 23% ee, which is slightly higher than the reported values (see Supplementary Note 1)51. Moreover, diethyl acetal 1k reacted smoothly with 2a to give the biaryl product 3k in 71% yield. IQMA 1a was coupled with 2-naphthols bearing electron-withdrawing or donating groups 2b–d at the 6-position to afford the corresponding 2-amino-2′-hydroxy-products 3l–n with 74–85% yields. The reactions using 3-methoxy- and 3-carboxyl-2-naphthols 2e and 2f afforded the coupling products 3o and 3p in 68 and 83% yields, respectively. Vinyloxy- (2g), tert-butyl dimethyl siloxy- (2h), and pinacol boronic ester groups (2i) at the 7-position were also tested into the flow system, giving the desired products 3q–s in 68–82% yields. Using 5-bromoresorcinol 2j as a phenolic nucleophile, the coupling product 3t was isolated in 67% yield.
Although a cross-coupling reaction using quinone monoacetal 4a and 2j for further extension of the substrate scope was performed under the optimized conditions, the isolated yield of the desired biarenol 5a was only 38%. Thus, to determine the appropriate reaction conditions for QMAs, BO-assisted screening of 4a and 2j as model substrates was performed (Table 2).
The experimental dataset (Table 2, entries 1–6) was collected under the same reaction conditions listed in Table 1 (entries 1–6). Similarly, when BO with parallel LCB and experimental evaluation were repeatedly performed (entries 7–15), the yield of product 5a was improved to 69% (isolated yield: 66%) with a use of β-type mixer conditions in entry 15. In the previous reaction (Table 1), different mixer (Comet-X), and lower concentration of IQMAs 1 was required compared to the optimized conditions (Table 2). When we tested this lower concentration using QMAs 4, low conversion of 4 was observed, while testing IQMAs 1 under these new conditions generated many side products. Hence the difference in the mixer suitable for each reaction can be rationalized to be due to the difference in the respective stirring methods. Under the revised reaction conditions (Table 2, entry 15), we evaluated the scope of the cross-coupling reaction using QMAs 4 and arenols 2, according to Fig. 3. Using acceptor 4a with 5-methylresorcinol (2k) and non-substituted resorcinol (2l), the corresponding products 5b and 5c, were obtained in moderate yields (59 and 62%, respectively). Substrates such as 3,5-dimethylphenol (2m) and sesamol (2n) were successfully converted to 5d and 5e in 74 and 84% yields, respectively. When a variety of naphthols (2a: 2-naphthol; 2o: 6-cyano-2-naphthol; 2p: 6-bromo-2-naphthol; 2q: 7-methoxy-2-naphtol; 2r: 1-naphthol) reacted, the corresponding biarenols 5f–j were produced with 58–93% yields. The structure of 5j was further confirmed by X-ray crystallographic analysis (see Supplementary Note 6). In addition, the reaction of 3-methylated QMA 4b with 2a and 2n afforded the corresponding products in high yields (5k: 97% and 5l: 92%). Finally, we found that sterically hindered 3,5-dimethylated 4c was tolerated in this transformation to give biarenols 5m and 5n in good yields (73 and 72%, respectively).
Yields are those of isolated 5. aReaction conditions: 4 (0.065 mmol), 2 (3.2 equiv.), and TfOH (0.35 mol%) in degassed dry toluene/AcOEt (conc. of 4, 0.044 M, 10/1), micro mixer: β-type, flow rate: 0.068 mL/min, at 30 °C. bFlow rate: 0.055 mL/min, at 35 °C. cTfOH (0.5 mol%), flow rate: 0.048 mL/min, at 35 °C.
Gram-scale synthesis and transformation of product
To demonstrate the utility of our reaction, we investigated the scalability of the reaction system. Using 1 gram of 1a under BO-established suitable conditions, the desired biaryl 3a was obtained with a yield of 85% (Fig. 4a). According to a previously reported procedure51, the methanesulfonyl group in 3e was successfully removed, and the corresponding product 6 was obtained with 86% yield (Fig. 4b) (see Supplementary Note 2). Moreover, the Ni(II)/Zn-catalyzed reductive coupling of 5f with diphenyl phosphine oxide provided the P-arylated product 7 with 60% yield (Fig. 4c)54.
Plausible reaction mechanism
A plausible reaction mechanism for the Brønsted acid-catalyzed cross-coupling reaction may involves the formation of a mixed acetal/[3,3]-sigmatropic rearrangement (Fig. 5)51. Initially, the strong Brønsted acid, TfOH, is thought to promote the formation of a mixed acetal from 1a and 2a. Subsequently, the [3,3]-sigmatropic rearrangement of the mixed acetal and re-aromatization would provide the biarenol 3a. According to Kürti’s report, we performed the coupling reaction of 2-methoxynaphthalene with 1a (see Fig. S3 in Supplementary Note 5) and the desired coupling product was not obtained because the methyl-capped substrate could not form the mixed acetal intermediate under our optimized reaction conditions. This suggests that our flow reaction proceeded by a similar reaction intermediate with that of Kürti’s reaction51.
In conclusion, we demonstrated a highly efficient, rapid, and regioselective synthetic method for various biaryls utilizing the redox-neutral cross-coupling of iminoquinone monoacetals or quinone monoacetals with arenols in a flow system. The reaction proceeded smoothly at room temperature in an inexpensive and low toxicity toluene medium with 1 mol% TfOH. BO-assisted parallel screening with one-hot encoding successfully estimated suitable reaction conditions, including both numerical and categorical parameters. Our algorithm can screen for engineering variables such as the type of micromixer, providing a method for chemists that does not require complicated quantification or descriptors. The optimized conditions were successfully applied to gram-scale synthesis. To the best of our knowledge, this is the first report on the redox-free flow process for the synthesis of highly functionalized biaryls. Our group is currently investigating BO-assisted screening of multiple categorical parameters and the highly enantioselective synthesis of biaryls using an immobilized chiral catalyst in flow.
Methods
General methods
For synthetic details and analytical data of all reaction starting materials 1 and 4, see Supplementary Methods 1 and 2. For synthetic and analytical details of all atropisomeric biaryls 3 and 5, see Supplementary Methods 3 and 4. For NMR spectra see Supplementary Data 1.
General procedure for the biaryl synthesis of IQMAs 1 and arenol 2 using TfOH in flow system
As shown in Fig. 6, a flow microreactor system was dipped in oil bath to heat at 25 °C. A solution of 1a (0.065 mmol, 0.015 M) in toluene (2.2 mL, syringe 1), and a solution of 2a (0.195 mmol, 0.045 M) and TfOH (1.5 mol%) in toluene (2.2 mL, syringe 2) were introduced to the flow microreactor system by syringe pumps at a flow rate: 0.08 mL/min (see Fig. S1 in Supplementary Method 3). The resulting solution was passed through Comet X mixer (total volume = 2.4 mL, residence time = 15 min) and directly forwarded to the quenching saturated aq. NaHCO3 solution. After all the amount of toluene solutions were pumped, we pumped a fresh air to the flow microreactor at the same flow rate (0.08 mL/min) to avoid losing 1 reactor volume inside. Finally, the organic layer was extracted with EtOAc (15 mL × 3), dried over Na2SO4, concentrated in vacuo. The residue was purified by silica column chromatography (n-hexane/EtOAc) to afford 3a (93%, 25.4 mg).
Data availability
Additional data supporting the findings described in this manuscript are available in the Supplementary information and supplementary Data 1–3. The X-ray crystallographic coordinate for structures reported in this study have been deposited at the Cambridge Crystallographic Data Center (CCDC) under deposition numbers CCDC-2142538 (5j). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the article and Supplementary information and Data 1–3, and also are available from the corresponding author upon reasonable request.
Code availability
Codes were deposited in a DOI-minting repository (Zenodo) under the title of ‘Scripts for categorical Bayesian optimization-assisted screening of reaction conditions in the flow biaryl synthesis’ https://doi.org/10.5281/zenodo.7151503.
References
Fantke, P. et al. Transition to sustainable chemistry through digitalization. Chem 7, 2866–2882 (2021).
Martinez Alvarado, J. I., Meinhardt, J. M. & Lin, S. Working at the interfaces of data science and synthetic electrochemistry. Tetrahedron Chem. 1, 100012 (2022).
Hardian, R., Liang, Z., Zhang, X. & Szekely, G. Artificial intelligence: The silver bullet for sustainable materials development. Green. Chem. 22, 7521–7528 (2020).
Dörr, M., Hielscher, M. M., Proppe, J. & Waldvogel, S. R. Electrosynthetic screening and modern optimization strategies for electrosynthesis of highly value-added products. ChemElectroChem 8, 2621–2629 (2021).
Vámosi, P. et al. Rapid optimization of reaction conditions based on comprehensive reaction analysis using a continuous flow microwave reactor. Chem. Rec. 19, 77–84 (2019).
Reizman, B. J. & Jensen, K. F. Feedback in flow for accelerated reaction development. Acc. Chem. Res. 49, 1786–1796 (2016).
Sans, V. & Cronin, L. Towards dial-a-molecule by integrating continuous flow, analytics, and self-optimisation. Chem. Soc. Rev. 45, 2032–2043 (2016).
Fitzpatrick, D. E., Maujean, T., Evans, A. C. & Ley, S. V. Across-the-world automated optimization and continuous-flow synthesis of pharmaceutical agents operating through a cloud-based server. Angew. Chem., Int. Ed. 57, 15128–15132 (2018).
Bedard, A.-C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361, 1220–1225 (2018).
Vasudevan, N. et al. Direct C-H arylation of indole-3-acetic acid derivatives enabled by an autonomous self-optimizing flow reactor. Adv. Synth. Catal. 363, 791–799 (2020).
Chatterjee, S., Guidi, M., Seeberger, P. H. & Gilmore, K. Automated radial synthesis of organic molecules. Nature 579, 379–384 (2020).
Gioiello, A., Piccinno, A., Lozza, A. M. & Cerra, B. The medicinal chemistry in the era of machines and automation: Recent advances in continuous flow technology. J. Med. Chem. 63, 6624–6647 (2020).
Sagandira, C. R. et al. Towards 4th industrial revolution efficient and sustainable continuous flow manufacturing of active pharmaceutical ingredients. React. Chem. Eng. 7, 214–244 (2022).
Breen, C. P., Nambiar, A. M. K., Jamison, T. F. & Jensen, K. F. Ready, set, flow! Automated continuous synthesis and optimization. Trends Chem. 3, 373–386 (2021).
Kondo, M. et al. Exploration of flow reaction conditions using machine-learning for enantioselective organocatalyzed Rauhut–Currier and [3+2] annulation sequence. Chem. Commun. 56, 1259–1262 (2020).
Schweidtmann, A. M. et al. Machine learning meets continuous flow chemistry: Automated optimization towards the Pareto front of multiple objectives. Chem. Eng. J. 352, 277–282 (2018).
Felton, K. C., Rittig, J. G. & Lapkin, A. A. Summit: Benchmarking machine learning methods for reaction optimisation. Chem.−Methods 1, 116–122 (2021).
Sagmeister, P. et al. Autonomous multi-step and multi-objective optimization facilitated by real-time process analytics. Adv. Sci. 9, 2105547 (2022).
Nambiar, A. M. K. et al. Bayesian optimization of computer-proposed multistep synthetic routes on an automated robotic flow platform. ACS Cent. Sci. 8, 825–836 (2022).
Sugisawa, N. et al. Rapid and mild one-flow synthetic approach to unsymmetrical sulfamides guided by Bayesian optimization. Chem.−Methods 1, 484–490 (2021).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Kondo, M. et al. Energy-, time-, and labor-saving synthesis of α-ketiminophosphonates: Machine-learning-assisted simultaneous multiparameter screening for electrochemical oxidation. Green. Chem. 23, 5825–5831 (2021).
Naito, Y. et al. Bayesian optimization with constraint on passed charge for multiparameter screening of electrochemical reductive carboxylation in a flow microreactor. Chem. Commun. 58, 3893–3896 (2022).
Zhang, Y., Apley, D. W. & Chen, W. Bayesian optimization for materials design with mixed quantitative and qualitative variables. Sci. Rep. 10, 4924 (2020).
Zhang, Y., Tao, S., Chen, W. & Apley, D. W. A latent variable approach to gaussian process modeling with qualitative and quantitative factors. Technometrics 62, 291–302 (2020).
Häse, F., Aldeghi, M., Hickman, R. J., Roch, L. M. & Aspuru-Guzik, A. Gryffin: An algorithm for Bayesian optimization of categorical variables informed by expert knowledge. Appl. Phys. Rev. 8, 031406 (2021).
Ehrfeld, W., Golbig, K., Hessel, V., Löwe, H. & Richter, T. Characterization of mixing in micromixers by a test reaction: single mixing units and mixer arrays. Ind. Eng. Chem. Res. 38, 1075–1082 (1999).
Guichardon, P. & Falk, L. Characterisation of micromixing effciency by the iodide/iodate reaction system. Part II: Kinetic Study. Chem. Eng. Sci. 55, 4233–4243 (2000).
Panić, S., Loebbecke, S., Tuercke, T., Antes, J. & Bošković, D. Experimental approaches to a better understanding of mixing performance of microfluidic devices. Chem. Eng. J. 101, 409–419 (2004).
Hessel, V. Novel process windows - gate to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 32, 1655–1681 (2009).
Kashid, M., Renken, A. & Kiwi-Minsker, L. Mixing efficiency and energy consumption for five generic microchannel designs. Chem. Eng. J. 167, 436–443 (2011).
Gobert, S. R. L., Kuhn, S., Braeken, L. & Thomassen, L. C. J. Characterization of milli- and microflow reactors: Mixing efficiency and residence time distribution. Org. Process Res. Dev. 21, 531–542 (2017).
A Bayesian Optimization framework in python https://github.com/SheffieldML/GPyOpt (2016).
Kondo, M. et al. Scripts for categorical Bayesian optimization-assisted screening of reaction conditions in the flow biaryl synthesis. Zenodo. https://doi.org/10.5281/zenodo.7151503 (2022).
Aldemir, H., Richarz, R. & Gulder, T. A. M. The biocatalytic repertoire of natural biaryl formation. Angew. Chem. Int. Ed. 53, 8286–8293 (2014).
Tajuddeen, A. & Bringmann, G. N, C-Coupled naphthylisoquinoline alkaloids: A versatile new class of axially chiral natural products. Nat. Prod. Rep. 38, 2145–2186 (2021).
Clayden, J., Moran, W. J., Edwards, P. J. & LaPlante, S. R. The challenge of atropisomerism in drug discovery. Angew. Chem., Int. Ed. 48, 6398–6401 (2009).
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A. & Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 6, 505–513 (2011).
LaPlante, S. R. et al. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 54, 7005–7022 (2011).
Chen, Y., Yekta, S. & Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 103, 3155–3212 (2003).
Brunel, J. M. BINOL: A versatile chiral reagent. Chem. Rev. 105, 857–898 (2005).
Li, Y.-M., Kwong, F.-Y., Yu, W.-Y. & Chan, A. S. C. Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev. 251, 2119–2144 (2007).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: History and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Zhang, Y. F. & Shi, Z. J. Upgrading cross-coupling reactions for biaryl syntheses. Acc. Chem. Res. 52, 161–169 (2019).
Wang, H. Recent advances in asymmetric oxidative coupling of 2-naphthol and its derivatives. Chirality 22, 827–837 (2010).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Sako, M., Takizawa, S. & Sasai, H. Chiral vanadium complex catalyzed oxidative coupling of arenols. Tetrahedron 76, 131645 (2020).
Niederer, K. A., Gilmartin, P. H. & Kozlowski, M. C. Oxidative photocatalytic homo- and cross-coupling of phenols: Nonenzymatic, catalytic method for coupling tyrosine. ACS Catal. 10, 14615–14623 (2020).
Hayashi, H., Ueno, T., Kim, C. & Uchida, T. Ruthenium-catalyzed cross-selective asymmetric oxidative coupling of arenols. Org. Lett. 22, 1469–1474 (2020).
Dyadyuk, A. et al. A chiral iron disulfonate catalyst for the enantioselective synthesis of 2‑amino-2’-hydroxy-1,1’-binaphthyls (NOBINs). J. Am. Chem. Soc. 144, 3676–3684 (2022).
Gao, H. et al. Practical organocatalytic synthesis of functionalized non-C2-symmetrical atropisomeric biaryls. Angew. Chem., Int. Ed. 55, 566–571 (2016).
Morimoto, K., Sakamoto, K., Ohshika, T., Dohi, T. & Kita, Y. Organo-iodine(III)-catalyzed oxidative phenol–arene and phenol–phenol cross-coupling reaction. Angew. Chem., Int. Ed. 55, 3652–3656 (2016).
Gonzalez, J., Dai, P., Hennig, P. & Lawrence, N. Batch Bayesian optimization via local penalization. Proc. 19th Int. Conf. Artif. Intell. Stat. 51, 648–657 (2016).
Zhang, X. et al. Ni(II)/Zn catalyzed reductive coupling of aryl halides with diphenylphosphine oxide in water. Org. Lett. 13, 3478–3481 (2011).
Acknowledgements
This study was funded by grants from JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (No. 22K06502), Transformative Research Areas (A) 21A204 Digitalization-driven Transformative Organic Synthesis (Digi-TOS) (No. 21H05207 and 21H05217) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and the Japan Society for the Promotion of Science (JSPS), JST CREST (No. JPMJCR20R1), JST CREST(No. JPMJCR1666), Hoansha Foundation, and Kansai Research Foundation for Technology Promotion. Dr. Takahide Fukuyama is acknowledged for his useful advice on micromixers. We acknowledge the technical staff of the Comprehensive Analysis Center of SANKEN, Osaka University (Japan).
Author information
Authors and Affiliations
Contributions
M.K.: Designing the experiments and writing the paper. H.D.P.W. and M.S.H.S.: Performing the experiments and analyzing data. K.I., S.H., T.T., and T.W.: Creating and modification of GPR scripts. S.T. and H.S.: Supervising the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kondo, M., Wathsala, H.D.P., Salem, M.S.H. et al. Bayesian optimization-driven parallel-screening of multiple parameters for the flow synthesis of biaryl compounds. Commun Chem 5, 148 (2022). https://doi.org/10.1038/s42004-022-00764-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00764-7
This article is cited by
-
Continuous flow process optimization aided by machine learning for a pharmaceutical intermediate
Journal of Flow Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.