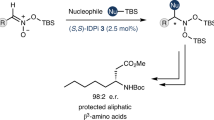
Abstract
The asymmetric cyanation of acylsilanes affords densely functionalized tetrasubstituted chiral carbon centers bearing silyl, cyano, and hydroxy groups, which are of particular interest in synthetic and medicinal chemistry. However, this method has been limited to a few enzymatic approaches, which employ only one substrate because of substrate specificity. Here we show the non-enzymatic catalytic asymmetric cyanation of acylsilanes using a chiral Lewis base as an enantioselective catalyst, trimethylsilyl cyanide as a cyanating reagent, and isopropyl alcohol as an additive to drive catalyst turnover. High enantio- and site-selectivities are achieved in a catalytic manner, and a variety of functional groups are installed in optically active acylsilane cyanohydrins, thus overcoming the limitations imposed by substrate specificity in conventional enzymatic methods. A handle for the synthetic application of the products is also established through the development of a catalyst for protecting acylsilane cyanohydrins, which are unstable and difficult to protect alcohols.
Similar content being viewed by others
Introduction
The catalytic asymmetric cyanation of ketones constitutes a straightforward method for the construction of tetrasubstituted chiral carbon centers1,2,3,4,5,6,7,8,9,10,11,12,13,14,15, which are of particular interest in synthetic16,17,18,19,20,21,22,23 and medicinal24,25,26 chemistry. Indeed, owing to the utility of optically active tertiary alcohols bearing cyano groups27,28,29,30,31,32, significant advances have been made in their asymmetric synthesis1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18. The asymmetric cyanation of acylsilanes affords densely functionalized tetrasubstituted chiral carbon centers bearing silyl, cyano, and hydroxy groups. However, this method has been limited to a few enzymatic approaches, which employ only one substrate because of substrate specificity (Fig. 1a)33,34.
Owing to the increasing interest in chiral silicon-containing molecules, such as silicon isosteres in drug design and development35,36,37,38,39,40,41 and synthetic building blocks in stereocontrolled C–C bond formation and rearrangements42,43,44,45, there have been some recent reports on the catalytic asymmetric addition of carbon and heteroatom nucleophiles to acylsilanes46,47,48,49,50,51,52. However, among carbon nucleophiles, only alkylation and alkynylation have been reported to date (Fig. 1b)46,47,48,49,50,51. It is plausible that cyanation is hindered by the competing Brook rearrangement, which takes place rapidly in basic media44,53,54,55,56,57,58. As an alternative synthetic approach to optically active acylsilane cyanohydrins, we recently reported the kinetic resolution of chiral cyanohydrins generated in situ from acylsilanes involving organocatalytic asymmetric cyclization under nearly neutral conditions which prevent the occurrence of the Brook rearrangement (Fig. 1c)59. This reaction is to the best of our knowledge the first non-enzymatic catalytic asymmetric approach to the synthesis of optically active acylsilane cyanohydrins. However, the following problems remain: (1) the maximum yield is ca. 50% due to the principle of kinetic resolution; (2) the substrate structures are limited as they are required to undergo a 6-membered ring formation via intramolecular oxy-Michael addition. To solve these issues, it is necessary to develop a non-enzymatic approach to the asymmetric cyanation of acylsilanes via enantioselective 1,2-addition reactions. Organocatalysis is an effective method not only for achieving high enantioselectivity but also for preventing side reactions. Therefore, we designed a chiral amine-catalyzed cyanation using a silyl cyanide (Fig. 1d)8,11. In addition, it is important to establish protocols for transforming the resulting cyanohydrin products, because they remain susceptible to the Brook rearrangement and are difficult to protect because of the adjacent bulky silyl group. In this study, we developed a novel Lewis-base-catalyzed enantioselective cyanation of acylsilanes, which is to the best of our knowledge the first non-enzymatic catalytic method that leads to quantitative yields of optically active acylsilane cyanohydrins. This method does not necessitate any specific substrate structure, and various functional groups are tolerated. Furthermore, the newly developed catalytic method for the silylation of the product alcohols constitutes a valuable handle for their synthetic applications.
Results
Optimization of reaction conditions
We initiated our investigations using 4-phenyl-1-(trimethylsilyl)butan-1-one (1a) and 2.0 equivalents of trimethylsilyl cyanide (TMSCN) with 20 mol % of the chiral amine catalysts 3a–3h (Fig. 2) in CHCl3 at –78 °C (Table 1, entries 1–8; see also Supplementary Table 1). Catalyst 3a yielded the corresponding product 2a with high enantioselectivity (Table 1, entry 1), while the cinchona alkaloids 3b–3e and other amine catalysts 3f and 3g were less active and exhibited lower enantioselectivity (Table 1, entries 2–7). The use of catalyst 3h resulted in a high yield but low enantioselectivity. In addition, while the catalysts 3a–3g afforded the alcohol product 2a, only catalyst 3h yielded the trimethylsilyl ether of 2a. Various solvents were also investigated using 3a as the catalyst (Table 1, entries 9–14). CHCl3 proved to be the most efficient solvent (Table 1, entry 1). At a lower catalyst loading of 10 mol %, a significantly low yield was obtained, while a high enantioselectivity was maintained (Table 1, entry 15). Lengthening the reaction time did not improve the yield (Table 1, entry 16), which implied that the catalyst was deactivated during the reaction.
As the product 2a was obtained as the alcohol, it was postulated that the silyl group derived from TMSCN remained attached to the Lewis basic moiety of 3a after the cyanation of 1a, which disturbed the turnover of 3a. Therefore, to improve the catalytic efficiency, the use of an additive was investigated (Table 2; see also Supplementary Table 2). Alcohols were used as additives for scavenging the silyl group and protonating the alkoxide resulting from the cyanation (in the absence of any additive, the dissolved water was probably involved in the catalytic cycle to yield the product, albeit in low yields). In the presence of methanol (MeOH), although the yield significantly increased as expected, the enantioselectivity decreased (Table 2, entry 2). A bulkier alcohol, isopropyl alcohol (i-PrOH), provided a higher enantioselectivity than MeOH, while the yield was similarly high (Table 2, entry 3). However, an even bulkier alcohol, tertiary butyl alcohol, exhibited negligible effects as an additive (Table 2, entry 4). Further investigations revealed that the use of 1.0 equivalent of i-PrOH with 5.0 mol % of 3a provided a high yield with appreciable enantioselectivity (Table 2, entry 5). A 1.0 mmol scale reaction also resulted in comparable results (see Supplementary Scheme 1 for details). Higher temperatures resulted in lower enantioselectivities (see Supplementary Table 2 for details).
Mechanistic investigations
The alcohol additives play two possible roles. First, i-PrOH scavenges the silyl group remaining on the Lewis basic moiety of 3a after the cyanation of 1a, which involves the formation of a 3a-TMSCN complex as the cyanating species. Second, i-PrOH reacts with TMSCN to supply hydrogen cyanide (HCN), which is subsequently activated by 3a for the cyanation of 1a. The formation of these species was verified by performing nuclear magnetic resonance (NMR) analyses of the reaction mixtures (Fig. 3). NMR analysis of the solution of 3a and TMSCN in CDCl3 (reaction time: 15 min) indicated that the signal associated with the protons of the TMS group was shifted upfield, suggesting the coordination of 3a to the silyl group of TMSCN (Fig. 3a; see also Supplementary Scheme 2). The solution of i-PrOH and TMSCN in CDCl3 (reaction time: 6 h) exhibited only a small signal associated with i-PrOTMS, which appeared along with the generation of HCN, suggesting that in the absence of 3a, insignificant amounts of HCN were generated at low temperatures even after a long time (Fig. 3b; see also Supplementary Scheme 3). On the other hand, the solutions of MeOH and i-PrOH with TMSCN and 3a in CDCl3 (reaction time: 15 min) exhibited signals associated with MeOTMS and i-PrOTMS, respectively, while those corresponding to the alcohols disappeared, suggesting that in the presence of 3a, HCN was generated in both the cases after a certain time (Fig. 3c and d; see also Supplementary Scheme 4).
a TMSCN:3a = 1:1. b TMSCN:i-PrOH = 1:1. c TMSCN:MeOH:3a = 2:1:0.05; the generation of TMSOTMS was also observed in 12% yield. d TMSCN:i-PrOH:3a = 2:1:0.05; the generation of TMSOTMS was also observed in 18% yield. The reaction mixtures were stirred in CDCl3 at –78 °C, and NMR analyses were carried out at –60 °C to prevent the solutions from freezing in the NMR sample tubes. Yields are values calculated with 1.0 equivalent of starting material, identified as 100%, and the theoretical maximum yield of TMSCN recovered under the conditions described in equations c and d is 200%.
Notably, the enantioselectivity was affected by the alcohol additive (Table 2, entries 2 and 3), and we supposed the existence of two competing catalytic pathways, which involved the 3a-TMSCN complex and the 3a-HCN complex as the cyanating species, in a parallel manner. We hypothesized that the pathway involving 3a-TMSCN afforded a higher enantioselectivity than the one involving 3a-HCN, and i-PrOH produced HCN at a slower rate than MeOH. Thus, we tested a modified procedure, in which a mixture of i-PrOH, TMSCN, and 3a was stirred in CHCl3 at –78 °C for 30 min, which was sufficient to generate HCN, before 1a was added (Fig. 4). Under these conditions, the enantioselectivity decreased to 82% enantiomeric excess (ee), which was even less than that of the reaction using MeOH with the optimized procedures (86% ee). These results support the hypothesis mentioned above.
The catalytic pathways are proposed based on the experimental results (Fig. 5; see also Supplementary Scheme 5). The 3a-TMSCN complex is generated as a common intermediate. It may react rapidly with less bulky alcohols (e.g. MeOH) to provide HCN, which is involved in the less enantioselective catalytic cycle (3a-HCN pathway). On the other hand, the reaction of i-PrOH with the 3a-TMSCN complex is slow, which is probably due to the bulkiness of i-PrOH, and the 3a-TMSCN complex is involved in the cyanation (3a-TMSCN pathway), thereby leading to a higher enantioselectivity. In the 3a-TMSCN pathway, i-PrOH scavenges the TMS group from 3a-TMSCN-1 to regenerate 3a and protonates the resulting alkoxide to provide 2. Experiments using i-PrOH-d8 are also mentioned in Supplementary Scheme 6.
Substrate scope and site-selectivity
We explored the substrate scope under the optimized conditions using 5.0 mol % of 3a and 1.0 equivalent of i-PrOH (Fig. 6). Various silyl groups were investigated (Figs. 6, 2a–e). Bulky silyl groups were found to provide higher enantioselectivities but lower yields. A bulky alkyl group was also tolerated, providing moderate enantioselectivity, albeit with a low yield (Figs. 6, 2f). A shorter alkyl group was well tolerated, affording the product in good yield with high enantioselectivity (Figs. 6, 2g). We also investigated substrates bearing various functional groups on the alkyl group (Figs. 6, 2h–p). Halogenated substrates resulted in good yields and enantioselectivities (Figs. 6, 2h and i). Ester, thioester, and sulfonic ester functionalities, which are useful for further transformations, were also tolerated to afford the corresponding products with high enantioselectivities (Figs. 6, 2j, k, and l). In addition, amino and amide group-bearing substrates afforded good yields with high enantioselectivities (Figs. 6, 2m and n). Terminal alkenyl and alkynyl groups, which are useful not only as tags for imaging60,61 and ligation62,63 for chemical biology studies but also as platforms for further functionalization64,65, also participated in the reaction to afford the products in high yields and with high enantioselectivities (Figs. 6, 2o and p). Actually, 2p was transformed via copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC)62,63,66 in high yield without erosion of its optical purity, which demonstrated the utility of click ligation for the accumulation of functional structures on the obtained products (Fig. 7). Other substrates we investigated are also mentioned in Supplementary Scheme 7.
Furthermore, the site-selectivity between different carbonyl groups was demonstrated (Fig. 8). The acylsilane cyanohydrins 2q and 2r were obtained in high yields and with good enantioselectivities with intact methyl ketone moieties, which are difficult to achieve using conventional catalysis for the cyanation of ketones (Fig. 8a)1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. This was attributed to the high electrophilicity of the acylsilanes67,68,69 in combination with the non-enzymatic but enzyme-like characteristics of the mild organocatalysts70. Moreover, a substrate bearing an enone moiety, which was used in our kinetic resolution approach (Fig. 1c)59, was site- and enantioselectively cyanated without cyclization as well as cyanation at the enone moiety to provide the optically active cyanohydrin 2s in a yield exceeding 50%, which demonstrates the utility of the current cyanation process involving enantioselective 1,2-addition (Fig. 8b). The absolute configuration of 2a was determined by X-ray crystallography (see Supplementary Fig. 129 and Supplementary Data 1 for details), and the configurations of all the other products were assigned analogously.
Catalytic silylation and transformations of the acylsilane cyanohydrin products
To transform acylsilane cyanohydrins, it is important to establish a method for protecting the alcohol moiety. As acylsilane cyanohydrins are unstable against the Brook rearrangement, mild conditions are required, and strongly basic conditions should be avoided. Additionally, as the bulky silyl groups of acylsilane cyanohydrins retard the protection (some conventional methods for alcohol protection were ineffective when applied on 2a; see Supplementary Scheme 8 for details), which is consistent with the formation of the non-silylated product 2a when cyanation was performed using the catalysts 3a–3g (Table 1, entries 1–7), an active catalyst is necessary for protection. According to entry 8 of Table 1, only sparteine (3h) afforded the cyanated product as a silyl ether. Inspired by these results, the sparteine-catalyzed silylation of the optically active acylsilane cyanohydrin was investigated (Table 3). As expected, the 3h-catalyzed silylation of 2a proceeded smoothly (Table 3, entry 1). Other diamines were also examined. Tetramethylethylenediamine (6) was inactive as the catalyst (Table 3, entry 2), and 1,8-bis(dimethylamino)naphthalene (7) provided a lower yield (Table 3, entry 3). In addition, other silylation reagents did not react in the presence of the catalyst 3h (Table 3, entries 4–6). Thus, the use of TMSCN in conjunction with the catalyst 3h was established as a reliable method for the protection of the acylsilane cyanohydrins. The product 5a was obtained in 97% yield upon lowering the catalyst loading at –40 °C (Table 3, entry 7). Moreover, the optical purity of the optically active compound 2a was maintained during the reaction (Table 3, entry 8; see also Supplementary Scheme 9). This method proved to be effective not only at –40 °C (Table 3, entry 8), but also at an ambient temperature (Table 3, entry 9) and produced 5a in a quantitative yield without any side-reaction while maintaining the enantiomeric purity. Therefore, the method is synthetically versatile depending on the stability and reactivity of the alcohol substrates (see also Supplementary Scheme 10).
The transformation of the unprotected cyanohydrin 2a and protected cyanohydrin 5a was demonstrated through the synthesis of the corresponding amides using two established hydration methods (Fig. 9)71,72. When the method using acetamide with palladium nitrate as a catalyst was employed71, the nitrile 2a was transformed to the amide 8 in 76% yield while maintaining the enantiomeric purity without the formation of the Brook side-product 9 due to the acidic conditions (Fig. 9a). On the other hand, when 2a was subjected to less acidic conditions, which is desirable for the synthesis of some multifunctional molecules, using acetaldoxime with indium chloride as a catalyst72, the Brook side-product 9 was obtained in 76% yield without the formation of 8 (Fig. 9b). The latter method also allowed the transformation of the nitrile 5a to the amide 10 without the loss of enantiomeric purity, albeit with a low conversion ratio under the current conditions, and the Brook rearrangement was completely suppressed (Fig. 9c). These facts indicate that the sparteine-catalyzed silylation outlined in Table 3 further expands the synthetic utility of the optically active acylsilane cyanohydrins, a variety of which are now available through the 3a-catalyzed cyanation developed in this study.
Conclusion
In summary, the non-enzymatic catalytic asymmetric cyanation of acylsilanes was accomplished using the chiral Lewis base as the catalyst, TMSCN as the cyanating reagent, and i-PrOH as the additive to drive catalyst turnover. High enantio- and site-selectivities were achieved in a catalytic manner, and a variety of functional groups were installed in the optically active acylsilane cyanohydrins, which overcame the limitations imposed by substrate specificity in conventional enzymatic methods. Moreover, a handle for the synthetic application of the products was established through the development of catalytic methods for the silylation of unstable and difficult to protect alcohols. These synthetic methods provide tetrasubstituted chiral carbon centers integrating multiple functional groups, including silyl, cyano, hydroxy, and functionalized alkyl groups. An efficient catalytic approach was thus developed for the preparation of potential building blocks for the synthesis of pharmaceutically relevant chiral organosilanes.
Methods
General procedure for aymmetric cyanation of acylsilanes 1
To a 5-mL vial were sequentially added acylsilane 1 (0.20 mmol), CHCl3 (0.10 mL), TMSCN (50 μL, 0.40 mmol), and i-PrOH (15 μL, 0.20 mmol). After the reaction mixture was stirred at –78 °C for 15 min, a solution of 3a (8.6 mg, 0.010 mmol) in CHCl3 (0.10 mL) was added. The mixture was stirred for 24 h. The reaction mixture was subsequently diluted with EtOAc, passed through a short silica gel pad, and concentrated in vacuo. Purification of the crude product by flash silica gel column chromatography using hexane/EtOAc (v/v = 2:1–20:1) as an eluent afforded the corresponding acylsilane cyanohydrin 2.
Procedure for trimetylsilylation of acylsilane cyanohydrin 2a
To a 5-mL vial were sequentially added acylsilane cyanohydrin 2a (58 mg, 0.20 mmol), CHCl3 (0.20 mL), and (–)-sparteine (2.3 mg, 0.010 mmol). After the reaction mixture was stirred at –40 °C for 30 min, TMSCN (50 μL, 0.40 mmol) was added. The mixture was stirred for 24 h. The reaction mixture was subsequently diluted with EtOAc, passed through a short silica gel pad, and concentrated in vacuo. Purification of the crude product by flash silica gel column chromatography using hexane/EtOAc (v/v = 20:1) as an eluent afforded the corresponding trimethylsilyl ether 5a.
Data availability
Additional data supporting the findings described in this manuscript are available in the Supplementary Information. For full characterization data of new compounds and experimental details, see Supplementary Methods. For the 1H and 13C NMR spectra of new compounds, see Supplementary Figs. 1–104. For HPLC chromatogram profiles of the reaction products, see Supplementary Figs. 105–128. For an ORTEP drawing of 2a, see Supplementary Fig. 129. For the cif, see Supplementary Data 1. X-ray crystallographic data have also been deposited in Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/) with the accession code CCDC 2112913. All other data are available from the authors upon reasonable request.
References
Kurono, N. & Ohkuma, T. Catalytic asymmetric cyanation reactions. ACS Catal. 6, 989–1023 (2016).
Murtinho, D. & da Silva Serra, M. E. Organocatalysed cyanations of carbonyl compounds. Curr. Organocatal 1, 87–106 (2014).
Wang, W., Liu, X., Lin, L. & Feng, X. Recent progress in the chemically catalyzed enantioselective synthesis of cyanohydrins. Eur. J. Org. Chem. 4751–4769 (2010).
Wang, J. et al. Asymmetric cyanation of aldehydes, ketones, aldimines, and ketimines catalyzed by a versatile catalyst generated from cinchona alkaloid, achiral substituted 2,2’-biphenol and tetraisopropyl titanate. Chem. —Eur. J. 15, 11642–11659 (2009).
Brunel, J.-M. & Holmes, I. P. Chemically catalyzed asymmetric cyanohydrin syntheses. Angew. Chem. Int. Ed. 43, 2752–2778 (2004).
Hamashima, Y., Kanai, M. & Shibasaki, M. Catalytic enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 122, 7412–7413 (2000).
Deng, H., Isler, M. P., Snapper, M. L. & Hoveyda, A. H. Aluminum-catalyzed asymmetric addition of TMSCN to aromatic and aliphatic ketones promoted by an easily accessible and recyclable peptide ligand. Angew. Chem. Int. Ed. 41, 1009–1012 (2002).
Tian, S.-K., Hong, R. & Deng, L. Catalytic asymmetric cyanosilylation of ketones with chiral Lewis base. J. Am. Chem. Soc. 125, 9900–9901 (2003).
Ryu, D. H. & Corey, E. J. Enantioselective cyanosilylation of ketones catalyzed by a chiral oxazaborolidinium ion. J. Am. Chem. Soc. 127, 5384–5387 (2005).
Fuerst, D. E. & Jacobsen, E. N. Thiourea-catalyzed enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 127, 8964–8965 (2005).
Liu, X., Qin, B., Zhou, X., He, B. & Feng, X. Catalytic asymmetric cyanosilylation of ketones by a chiral amino acid salt. J. Am. Chem. Soc. 127, 12224–12225 (2005).
Kurono, N., Uemura, M. & Ohkuma, T. Asymmetric cyanosilylation of α-keto esters catalyzed by the [Ru(phgly)2(binap)]-C6H5OLi system. Eur. J. Org. Chem. 1455–1459 (2010).
Ogura, Y., Akakura, M., Sakakura, A. & Ishihara, K. Enantioselective cyanoethoxycarbonylation of isatins promoted by a Lewis base-Brønsted acid cooperative catalyst. Angew. Chem. Int. Ed. 52, 8299–8303 (2013).
Zeng, X.-P. et al. Activation of chiral (salen)AlCl complex by phosphorane for highly enantioselective cyanosilylation of ketones and enones. J. Am. Chem. Soc. 138, 416–425 (2016).
Hatano, M., Yamakawa, K., Kawai, T., Horibe, T. & Ishihara, K. Enantioselective cyanosilylation of ketones with lithium(I) dicyanotrimethylsilicate(IV) catalyzed by a chiral lithium(I) phosphoryl phenoxide. Angew. Chem. Int. Ed. 55, 4021–4025 (2016).
Riant, O. & Hannedouche, J. Asymmetric catalysis for the construction of quaternary carbon centres: nucleophilic addition on ketones and ketimines. Org. Biomol. Chem. 5, 873–888 (2007).
Shibasaki, M. & Kanai, M. Catalytic enantioselective construction of tetrasubstituted carbons by self-assembled poly rare earth metal complexes. Org. Biomol. Chem. 5, 2027–2039 (2007).
Hatano, M. & Ishihara, K. Recent progress in the catalytic synthesis of tertiary alcohols from ketones with organometallic reagents. Synthesis 1647–1675 (2008).
Douglas, C. J. & Overman, L. E. Catalytic asymmetric synthesis of all-carbon quaternary stereocenters. Proc. Natl Acad. Sci. U. S. A. 101, 5363–5367 (2004).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2015).
Shockley, S. E., Holder, J. C. & Stoltz, B. M. Palladium-catalyzed asymmetric conjugate addition of arylboronic acids to α,β-unsaturated cyclic electrophiles. Org. Process Res. Dev. 19, 974–981 (2015).
Zeng, X.-P., Cao, Z.-Y., Wang, Y.-H., Zhou, F. & Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 116, 7330–7396 (2016).
Talele, T. T. Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 63, 13291–13315 (2020).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Yabu, K. et al. Switching enantiofacial selectivities using one chiral source: catalytic enantioselective synthesis of the key intermediate for (20S)-camptothecin family by (S)-selective cyanosilylation of ketones. J. Am. Chem. Soc. 123, 9908–9909 (2001).
Masumoto, S., Suzuki, M., Kanai, M. & Shibasaki, M. Catalytic asymmetric synthesis of (S)-oxybutynin and a versatile intermediate for antimuscarinic agents. Tetrahedron 60, 10497–10504 (2004).
Tamura, K., Furutachi, M., Kumagai, N. & Shibasaki, M. An enantioselective synthesis of voriconazole. J. Org. Chem. 78, 11396–11403 (2013).
Tamura, K., Kumagai, N. & Shibasaki, M. An enantioselective synthesis of the key intermediate for triazole antifungal agents; application to the catalytic asymmetric synthesis of efinaconazole (Jublia). J. Org. Chem. 79, 3272–3278 (2014).
Friedrich, K. & Wallenfels, K. The Chemistry of the Cyano Group (ed Rappoport, Z.) (Wiley-Interscience, New York, 1970).
Fatiadi, A. J. in The Chemistry of Triple-Bonded Functional Groups Vol. 2 (eds Patai, S. & Rappaport, Z.) Ch. 26 (John Wiley & Sons, Chichester, U. K., 1983).
Li, N., Zong, M.-H., Peng, H.-S., Wu, H.-C. & Liu, C. (R)-Oxynitrilase-catalyzed synthesis of (R)-2-trimethylsilyl-2-hydroxyl-ethylcyanide. J. Mol. Catal. B 22, 7–12 (2003).
Nanda, S., Kato, Y. & Asano, Y. A new (R)-hydroxynitrile lyase from Prunus mume: asymmetric synthesis of cyanohydrins. Tetrahedron 61, 10908–10916 (2005).
Showell, G. A. & Mills, J. S. Chemistry challenges in lead optimization: silicon isosteres in drug discovery. Drug Discov. Today 8, 551–556 (2003).
Meanwell, N. A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 54, 2529–2591 (2011).
Min, G. K., Hernández, D. & Skrydstrup, T. Efficient routes to carbon–silicon bond formation for the synthesis of silicon-containing peptides and azasilaheterocycles. Acc. Chem. Res. 46, 457–470 (2013).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Fujii, S. & Hashimoto, Y. Progress in the medicinal chemistry of silicon: C/Si exchange and beyond. Future Med. Chem. 9, 485–505 (2017).
Ramesh, R. & Reddy, D. S. Quest for novel chemical entities through incorporation of silicon in drug scaffolds. J. Med. Chem. 61, 3779–3798 (2018).
Barraza, S. J. & Denmark, S. E. Synthesis, reactivity, functionalization, and ADMET properties of silicon-containing nitrogen heterocycles. J. Am. Chem. Soc. 140, 6668–6684 (2018).
Lee, N., Tan, C.-H. & Leow, D. Asymmetric Brook rearrangement. Asian J. Org. Chem. 8, 25–31 (2019).
Moser, W. H. The Brook rearrangement in tandem bond formation strategies. Tetrahedron 57, 2065–2084 (2001).
Brook, A. G. Molecular rearrangements of organosilicon compounds. Acc. Chem. Res. 7, 77–84 (1974).
Eppe, G., Didier, D. & Marek, I. Stereocontrolled formation of several carbon–carbon bonds in acyclic systems. Chem. Rev. 115, 9175–9206 (2015).
Li, F.-Q., Zhong, S., Lu, G. & Chan, A. S. C. Zinc-salen-catalyzed asymmetric alkynylation of alkyl acylsilanes. Adv. Synth. Catal. 351, 1955–1960 (2009).
Smirnov, P. et al. One-pot zinc-promoted asymmetric alkynylation/Brook-type rearrangement/ene–allene cyclization: highly selective formation of three new bonds and two stereocenters in acyclic systems. Angew. Chem. Int. Ed. 52, 13717–13721 (2013).
Rong, J., Oost, R., Desmarchelier, A., Minnaard, A. J. & Harutyunyan, S. R. Catalytic asymmetric alkylation of acylsilanes. Angew. Chem. Int. Ed. 54, 3038–3042 (2015).
Han, M.-Y., Xie, X., Zhou, D., Li, P. & Wang, L. Organocatalyzed direct aldol reaction of silyl glyoxylates for the synthesis of α‑hydroxysilanes. Org. Lett. 19, 2282–2285 (2017).
Han, M.-Y., Luan, W.-Y., Mai, P.-L., Li, P. & Wang, L. Organocatalytic asymmetric vinylogous aldol reaction of allyl aryl ketones to silyl glyoxylates. J. Org. Chem. 83, 1518–1524 (2018).
Feng, J.-J. & Oestreich, M. Tertiary α-silyl alcohols by diastereoselective coupling of 1,3-dienes and acylsilanes initiated by enantioselective copper-catalyzed borylation. Angew. Chem. Int. Ed. 58, 8211–8215 (2019).
Guan, M. et al. Catalytic asymmetric addition of thiols to silyl glyoxylates for synthesis of multi-hetero-atom substituted carbon stereocenters. Chem. Sci. 12, 7498–7503 (2021).
Brook, A. G. Isomerism of some α-hydroxysilanes to silyl ethers. J. Am. Chem. Soc. 80, 1886–1889 (1958).
Takeda, K. & Ohnishi, Y. Reaction of acylsilanes with potassium cyanide: Brook rearrangement under phase-transfer catalytic conditions. Tetrahedron Lett. 41, 4169–4172 (2000).
Linghu, X., Nicewicz, D. A. & Johnson, J. S. Tandem carbon–carbon bond constructions via catalyzed cyanation/Brook rearrangement/C-acylation reactions of acylsilanes. Org. Lett. 4, 2957–2960 (2002).
Nicewicz, D. A., Yates, C. M. & Johnson, J. S. Enantioselective cyanation/Brook rearrangement/C-acylation reactions of acylsilanes catalyzed by chiral metal alkoxides. J. Org. Chem. 69, 6548–6555 (2004).
Nicewicz, D. A., Yates, C. M. & Johnson, J. S. Catalytic asymmetric acylation of (silyloxy)nitrile anions. Angew. Chem. Int. Ed. 43, 2652–2655 (2004).
Ando, M., Sasaki, M., Miyashita, I. & Takeda, K. Formation of 2-cyano-2-siloxyvinylallenes via cyanide-induced Brook rearrangement in γ-bromo-α,β,γ,δ-unsaturated acylsilanes. J. Org. Chem. 80, 247–255 (2015).
Matsumoto, A., Asano, K. & Matsubara, S. Kinetic resolution of acylsilane cyanohydrins via organocatalytic cycloetherification. Chem. —Asian J. 14, 116–120 (2019).
Yamakoshi, H. et al. Imaging of EdU, an alkyne-tagged cell proliferation probe, by raman microscopy. J. Am. Chem. Soc. 133, 6102–6105 (2011).
Yamakoshi, H. et al. Alkyne-tag raman imaging for visualization of mobile small molecules in live cells. J. Am. Chem. Soc. 134, 20681–20689 (2012).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
The Chemistry of Triple-Bonded Functional Groups Vol. 2 (eds Patai, S. &. Rappaport, Z.) (John Wiley & Sons, Chichester; New York; Brisbane; Toronto; Singapore, 1983).
The Chemistry of Double-Bonded Functional Groups Vol. 2 (ed Patai, S.) (John Wiley & Sons, Chichester; New York; Brisbane; Toronto; Singapore, 1989).
Shao, C. et al. Acid–base jointly promoted copper(I)-catalyzed azide–alkyne cycloaddition. J. Org. Chem. 76, 6832–6836 (2011).
Zhang, H.-J., Priebbenow, D. L. & Bolm, C. Acylsilanes: valuable organosilicon reagents in organic synthesis. Chem. Soc. Rev. 42, 8540–8571 (2013).
Wang, X. et al. Recent advances in the synthesis of acylsilanes. ChemCatChem 12, 5022–5033 (2020).
Page, P. C. B., Klair, S. S. & Rosenthal, S. Synthesis and chemistry of acyl silanes. Chem. Soc. Rev. 19, 147–195 (1990).
Asano, K. Multipoint recognition of molecular conformations with organocatalysts for asymmetric synthetic reactions. Bull. Chem. Soc. Jpn. 94, 694–712 (2021).
Kanda, T., Naraoka, A. & Naka, H. Catalytic transfer hydration of cyanohydrins to α‑hydroxyamides. J. Am. Chem. Soc. 141, 825–830 (2019).
Kim, E. S., Lee, H. S., Kim, S. H. & Kim, J. N. An efficient InCl3-catalyzed hydration of nitriles to amides: acetaldoxime as an effective water surrogate. Tetrahedron Lett. 51, 1589–1591 (2010).
Acknowledgements
We appreciate Professor Ryo Yazaki (Kyushu University) for his kind advice to our project. This work was supported financially by the Japanese Ministry of Education, Culture, Sports, Science and Technology (JP15H05845, JP17K19120, JP18K14214, JP18H04258, JP20K05491, and JP21H05076). K.A. also acknowledges the Institute for Synthetic Organic Chemistry, Toyo Gosei Memorial Foundation, the Sumitomo Foundation, Fukuoka Naohiko Memorial Foundation, Inoue Foundation for Science, Mizuho Foundation for the Promotion of Sciences, SPIRITS 2020 of Kyoto University, Japan Association for Chemical Innovation, and Tobe Maki Scholarship Foundation. T.N. and A.M. also acknowledges the Japan Society for the Promotion of Science for Young Scientists for the fellowship support (JP21J14481 and JP17J04451).
Author information
Authors and Affiliations
Contributions
K.A. conceived and designed the study. S.M. supervised the project. T.N., A.M., R.Y., and K.A. carried out the chemical experiments and analysed the data. K.A. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Chemistry thanks Jose Aleman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagano, T., Matsumoto, A., Yoshizaki, R. et al. Non-enzymatic catalytic asymmetric cyanation of acylsilanes. Commun Chem 5, 45 (2022). https://doi.org/10.1038/s42004-022-00662-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-022-00662-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.