Abstract
The mechanism of silver(I) and copper(I) catalyzed cycloaddition between 1,2-diazines and siloxy alkynes remains controversial. Here we explore the mechanism of this reaction with density functional theory. Our calculations show that the reaction takes place through a metal (Ag+, Cu+) catalyzed [2+2] cycloaddition pathway and the migration of a silylium ion [triisopropylsilyl ion (TIPS+)] further controls the reconstruction of four-member ring to give the final product. The lower barrier of this silylium ion mediated [2+2] cycloaddition mechanism (SMC) indicates that well-controlled [2+2] cycloaddition can obtain some poorly-accessible IEDDA (inverse-electron demand Diels-Alder reaction) products. Strong interaction of d10 metals (Ag+, Cu+) and alkenes activates the high acidity silylium ion (TIPS+) in situ. This п-acid (Ag+, Cu+) and hard acid (TIPS+) exchange scheme will be instructive in silylium ion chemistry. Our calculations not only provide a scheme to design IEDDA catalysts but also imply a concise way to synthesise 1,2-dinitrogen substituted cyclooctatetraenes (1,2-NCOTs).
Similar content being viewed by others
Introduction
Catalyzed [4+2] inverse-electron demand Diels–Alder reactions (IEDDA) remain challenging owing to biased activation of more electron-rich substrates1,2,3. Most catalytic approaches center on modulating two п-electron substrates for a better LUMO-HOMO matching according to FMO4. Lewis acid-catalyzed IEDDA between electro-rich alkynes (such as siloxy alkynes, ynamines) and aromatic diene (such as phthalazines) often requires relatively harsh conditions5,6.
[2+2] cycloaddition have been earned broad interest in recent years7,8. Four-member ring [2+2] cycloaddition product between alkynes and aromatic ring can undergo ring expansion to form cyclooctatetraene similarities (COTs), COTs can further undergo ring contraction and retro [2+2] to cover [4+2] Diels–Alder product9,10,11. Higashino and coworkers reported that substituted 1,2-diazines with ynamines were shown to proceed both [2+2] cycloaddition and [4+2] IEDDA cycloaddition, the [2+2] adduct followed with ring expansion to give diazacyclooctatetraene derivatives. They obtained [4+2] IEDDA product and diazacyclooctatetraene derivatives with comparable yield12,13 However, these schemes were limited by high reaction barrier and low chemo-selectivity14. To best our knowledge, there remains no report of [2+2] cycloaddition pathway which can exceed [4+2] Diels–Alder pathway to produce IEDDA products so far.
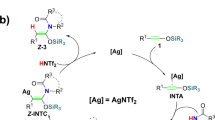
In 2012, Rawal and coworkers reported that silver (I) can efficiently catalyze phthalazines (sub1) and siloxy alkynes (sub2) to get 3-substituted 2-naphthol silyl ethers (3) in the room temperature when paired with 2,2-bipyridine as ligand. In 2014 they further discovered that copper(I) can supplant silver(I) to catalyze this reaction (Fig. 1a)15,16. For the strong Lewis acid (such as TiCl4, SnCl4, Sc(OTf)3 etc.) are ineffective of this reaction, they proposed that the high affinities of copper(I) and silver(I) to alkynes may take key roles in this reaction. They also found an interesting phenomenon that relatively electron richer ethoxy alkynes 2c and ynamide 2d (Fig. 1b) cannot react with phthalazines (sub1) in the same condition. The author proposed two pathways, the first pathway is coordination silver ion of phthalazines (sub1) rends it more electro deficiency (concerted [4+2] mechanism), and another plausible pathway which proceed through silver(I) induced nucleophilic attack of siloxy alkynes (sub2) to phthalazines (sub1) (stepwise [4+2] mechanism).
Recently Avcı et al. have explored silver(I) catalyzed [4+2] mechanism of this reaction17. Using a reduced model, they proposed the reaction can undergo through [4+2] stepwise mechanism with respect to ligand coordinated silver(I) (L-Ag+), sub1 and sub2 as reference point. With the same model and method, we found the barrier of [4+2] reaction may be underestimated in their work and [4+2] pathway is not favored in the room temperature(see details in Supplementary Note 1).
Note that there were quite many examples of d10 metal-induced nucleophilic reaction18,19,20. We proposed silylium ion [triisopropylsilyl ion (TIPS+)] mediated [2+2] cycloaddition (SMC) mechanism which accords with all experimental observations (Fig. 2a).
In our proposed silylium ion mediated [2+2] cycloaddition mechanism (SMC), break aromaticity of sub1 to form four-member ring int3 is essential step, which is a d10 metal [Ag(I) or Cu(I)] catalyzed [2+2] cycloaddition reaction. TIPS+ transfer to the tertiary amine of int3 further induce heterolysis of C-N bond which lead to metal coordinated ketene species int4a-1. Ketene species int4a-1 can easily undergo ring closure to form int5a which is very facile to release N2, TIPS+ and metal(I) phenate complex int7. TIPS+ combined with int7 gives final product.
As Rawal pointed, high affinity of d10 metals (Ag+ or Cu+) to alkynes and alkenes take crucial role in this reaction, which triggers the [2+2] cycloaddition reaction and migration of silylium ion (TIPS+) consecutively. If the silylium ion transfer step is inhibited and only [2+2] cycloaddition take place, this reaction can lead to four-member 11b H-Cyclobuta [2,1,α]phthalazine (CBP) which further undergo ring expansion to form 1,2-dinitrogen substituted eight-member ring 1,2-NCOT (Fig. 2b). 1,2-NCOT is interesting similarities of cyclooctatetraene (COT) and its reactivity remains almost unknown. This reaction may imply a very concise method to synthesis 1,2-NCOT which is kinetic stable according to our calculation. Our mechanism also provides an alternative solution for other alkynes which are inert to IEDDA, such as ynamines and ynamides etc5. If the protection group of ynamide 2d (Fig. 1b) changed into silicane group, this SMC pathway may extend to nitrogen-substituted alkynes. Silylium ion advantaged in its prominent high electrophilicity which showed excellent catalyze performance compared with traditional Lewis acid21,22,23,24,25,26,27. In this work, п-acidic d10 metals activate silylium ion in situ which then act as catalyst, this п-acid to hard acid scheme may also enlighten in silylium ion chemistry for most of silylium ion catalyzed reactions requires high acidity silylium ion prepared before reaction or generated in situ which need extremely active carbocation reagent22,23,28.
Results
Formation of Π-complexes M-int1(M = Ag or Cu)
In the silver case, coordination of 2,2′-bipyridine (bpy) to the catalyst salt AgOTf gives the bi-coordinated active center L-Ag+ is relatively exothermic by −23.1 kcal/mol (Fig. 3a). The next step is coordination of phthalazine 1a and siloxy alkynes 2a with bared active site L-Ag+ to obtain п-complex Ag-int1 which is exothermic by −30.3 kcal/mol.
In the copper(I) catalyzed IEDDA reaction, formation of п-complex Cu-int1 is very exergonic (−60.8 kcal/mol). The higher exothermic energy of formation Cu-int1 may be caused by relatively small diameter and high positive charge density of copper(I). Cu-int1 is more structurally compact compared with Ag-int1, and siloxy alkyne 2a coordinate more stronger with copper(I) atom (Fig. 3b). In both Cu-int1 and Ag-int1, the siloxy 2a significant deviations from linearity and in Cu-int1 triple bond C2-C3 elongate from 1.21 Å to 1.23 Å while in Ag-int1 C2-C3 bond elongate to 1.22 Å. The bond angle of ∠C1C2C3 twist from 180° to 163.37° in Cu-int1 and 170.6° in Ag-int1. Current literature have revealed quite a few well characterized copper(I) and silver(I) coordinated alkynes п-complexes and confirm that copper(I) coordinate stronger with alkynes than silver(I) in these п-complexes29. The H1 NMR and C13 NMR experiment also confirm evident interactions between siloxy alkynes with Ag(I) in DCM30.
Note that п-complexes Ag-in1 and Cu-int1 are the most stable intermediate before the reaction, we choose Ag-int1 and Cu-int1 as zero point for silver(I) and copper(I) catalyzed IEDDA respectively in the following discussion.
Silver catalyzed [2+2] Cycloaddition
Thermal constrains of orbital symmetry pose much challenge to direct [2+2] cycloaddition. Further, direct [2+2] cycloaddition of 1a and 2a needs to broke the aromaticity of the phthalazine ring. Our calculation shows that the barrier of this process as high as 54.6 kcal/mol. Transition metal offers the prospect of promoting the [2+2] cycloaddition through active alkynes by virtue of valence d-orbitals and lower energy of metallacyclic intermediates. Metal-catalyzed [2+2] reaction was also investigated by theory31,32.
Similar with most of metal-catalyzed cycloaddition reaction, nucleophilic addition of phthalazines 1a with siloxy alkene 2a activated by L-Ag+ is the initial step (Fig. 4a).
Our calculation shows that formation of ethylene silver species Ag-int2 is energetically accessible in the room temperature with a barrier (Ag-TS1) 19.5 kcal/mol (Fig. 4a), lower barrier of this process is mainly because the lone pair of oxygen atom in the Ag-TS1 conjugate with п orbitals of alkyne to stabilize the positive charge of silver(I). The NBO charge distribution shows the positive charge transferred from silver(I) to the nitrogen atom from Ag-int1 to Ag-int2 (N atom from −0.36 to −0.12, Ag atom 0.71 to 0.52). The phthalazine 1a remains nearly planar in the ethylene silver species Ag-int2, which indicate that the aromaticity of 1a is not completely destroyed in this step (Fig. 4b). An alternative step to obtain ethylene silver species occur via the attack of the siloxy alkyne from C2 point which is energetically unfavored with a barrier 32.4 kcal/mol.
The Ag-int2 transform into silver(I) coordinated four-member ring complex Ag-int3 is energetically accessible with a barrier (Ag-TS2) 14.3 kcal/mol. Compared with direct [2+2] cycloaddition between 1a and 2a, silver(I) catalyst can efficiently lower the barrier of this process (54.6 kcal/mol vs 19.5 kcal/mol). Experiment also confirmed that silver(I) and copper(I) can catalyze [2+2] cycloaddition of siloxy alkynes and substituted alkenes with quite good yield in room temperature16,30.
Silylium ion mediated four-member ring rearrangement (path a)
Direct migration of triisopropylsilyl ion (TIPS+) to form quaternary ammonium species Ag-int4a is energetically unfavored with a barrier (Ag-TS3a, see Supplementary Data 20) 31.9 kcal/mol. Silylium ion is notoriously unstable, and TIPS+ ion dissociate directly through SN1 pathway is also energetically unfavored with a barrier as high as 49.1 kcal/mol. However, against our chemical intuition, dissociate of TIPS+ through OTf− anion SN2 nucleophilic attack is quite easy with a barrier (Ag-TS3) only 14.9 kcal/mol (Fig. 5a). The high affinity of silver(I) with alkene anion compensate majority formation energy of TIPSOTf and made this process accessible with only 5.8 kcal/mol. The stabilized energy of L-Ag+ in the Ag-int4b as high as 33.1 kcal/mol. The strong interaction of silver(I) with alkene made Ag-int4b a very good leaving group. Commonly, weak Lewis base such as SO4−, OTf− are not good nucleophilic reagent, this situation changed when confront with good leaving group. Further, for the relative larger size of silicon atom compared with carbon atom, the steric protection effect of bulky substituents is less effective and can allow five coordinated structure, which means that many dissociative SN1-type reactions in organic chemistry can proceed through associative-SN2 mechanism with pentacoordinate transition state in silicon chemistry33.
L refers to 2,2’-bipyridine(bpy). Gibbs free energies are in kcal/mol. Hydrogen atom was omitted and carbon atom of ligand [2,2’-bipyridine(bpy)] was white cloaked for clarity. Triisopropylsilyl (TIPS) is simplified into big ball except desilication process and versa. a Reaction profile; b Optimized geometries.
TIPSOTf is regarded as anion stabilized silylium ion from a synthetic perspective, which is highly active reagent for amino and hydroxy protection. TIPS+ can easily transferred to nitrogen atom of tertiary amine in Ag-int4b to form quaternary ammonium species Ag-int4a through SN2 nucleophilic addition with a barrier (Ag-TS4a) 15.0 kcal/mol. In the quaternary ammonium species Ag-int4a, C-N bond is very easy to relax high tension of four-member ring and form silver(I) coordinated ketene species Ag-int4a-1 with a barrier (Ag-TS4a-1) only 0.1 kcal/mol. In this process positive charge transferred from nitrogen and silicon atoms (N-Si) to silver and carbon atoms (Ag-C). (NBO charge distribution shows that charge of N-Si change from 1.58 to 1.47, while Ag-C atoms change from 0.19 to 0.32). Ag-int4a-1 can easily undergo ring closure to form six-member Ag-int5a with a barrier (Ag-TS5a) 10.7 kcal/mol, the positive charge returns back from Ag-C to N-Si. (NBO charge distribution shows that charge of N-Si change from 1.47 to 1.79, and Ag-C change from 0.32 to 0.16). From four-member ring intermediate Ag-int4a to six-member ring intermediate Ag-int5a, there actually experience twice electron extraction competition between Ag-C and Si-N which is dominated by TIPS+ and L-Ag+. In the Ag-int4a the high positive charge of Si-N extracts the electron from electron-rich Ag-C bond, which lead to ring open of Ag-int4a to form Ag-int4a-1. The driving force of this step is high positive charge of Si-N and high tension of four-membered ring of Ag-int4a. The barrier from silver(I) coordinated ketene species Ag-int4a-1 to six-member ring Ag-int5a is 10.7 kcal/mol. Although high reactivity of ketene species and formation of N=N bond contribute to this step, this process is endothermic due to high Lewis acidity character of silylium ion, the driving force of this step is extreme high exothermic of release N2. Generally, release N2 is highly exothermic and go through concerted reaction pathway34,35. In this reaction, release N2 go through stepwise mechanism which can be attributed to high positive charge of silylium ion (TIPS+). In the Ag-int5a the length of C-N bond directly links to silylium ion (TIPS+) is 1.53 Å, another C-N bond is 1.45 Å, which is mainly caused by high positive charge of TIPS+. The relatively longer C-N bond induced by TIPS+ in the Ag-int5a indicate its relatively easy heterolysis character. Further, the electron-rich C-Ag bond can donate its electron to the C atom after heterolysis, this is very important driving force of C-N bond heterolysis. Actually, this heterolysis process is also an electron extraction competition between Ag-C and Si-N, the positive charge transferred from N-Si to C-Ag this time (NBO charge distribution shows that electron changed from 0.16 to 0.59 in C-Ag and 1.79 to 1.58 in N-Si from Ag-int5a to Ag-int6a). Because of these reasons, the barrier for heterolysis C-N bond and stepwise N2 release is only 2.9 kcal/mol. If there are no TIPS+ in this structure, the reaction can go through concerted mechanism with a barrier (see Ag-TS7b in Fig. 6b) 6.7 kcal/mol. The silylium ion stabilized dinitrogen intermediate Ag-int6a is very facile to release N2, TIPS+ and Ag-int7 (the negative free energy barrier of this step come from entropy and single point energy correction). TIPS+ combination with Ag-int7 to give final product is highly exothermic (−60.3 kcal/mol).
Ketone-enol tautomerism path way (path b)
In the Ag-int4b, the α-carbon is close to sp3 hybridization. The C-Ag-C angle is 121.9° (Fig. 5b), Like its α-H substituted ketone analogs, the intermediate Ag-int4b can go through ketone-enol tautomerism style intramolecular isomerization (Fig. 6) to obtain Ag-int5b-1 which is also the rate determine step of path b (23.9 kcal/mol). Direct dissociation of L-Ag+ from Ag-int4b is energetically unfavored with a barrier 33.1 kcal/mol (see Supplementary Fig. 4.).
Copper(I) catalyzed IEDDA with SMC mechanism
In the copper(I) catalyzed IEDDA, the rate determine barrier (Cu-TS1) of formation of four-member Cu-int3 is 26.8 kcal/mol (Fig. 7a), which is higher than silver(I) case (19.5 kcal/mol). After formation of Cu-int4b, only path a is energetically favored with a barrier 7.6 kcal/mol (Cu-TS4a), and path b is energetically unfavored with a rate determine barrier 33.8 kcal/mol (Fig. 7a, only key geometries was showed).
L refers to 2,2’-bipyridine(bpy). Gibbs free energies are in kcal/mol. The hydrogen atom was omitted and carbon atom of ligand [2,2’-bipyridine(bpy)] was white cloaked for clarity. Triisopropylsilyl (TIPS) is simplified into big ball except desilication process and versa. a Reaction profile; b Optimized geometries.
[4+2] reaction path way
Avcı et al. explored [4+2] mechanism with density functional theory (DFT), in their calculation, they simplified triisopropylsilyl (TIPS) group into trimethylsilyl (TMS) group and found that this reaction can proceeded through [4+2] stepwise mechanism17. We compared the rate determine barrier (RDB) of our proposed SMC mechanism with [4+2] stepwise mechanism under their model use different level of theory and found that the barrier of our proposed SMC mechanism lower than the [4+2] stepwise mechanism from 9.8 kcal/mol to 11.2 kcal/mol, which is a strong support that our proposed SMC mechanism is much more superior to [4+2] stepwise mechanism (see details in Supplementary Tables 1–3 and Supplementary Fig. 1). If we use the same reference point as their work (L-Ag+, 1a and 2a as reference point) and no reduced model, the barrier of silver catalyzed [4+2] mechanism is 26.1 kcal/mol which is also in accordance with their work.
In order to further illustrate that our proposed SMC mechanism is more suited to silver(I) and copper(I) catalyzed IEDDA reaction, we evaluated the barrier of traditional [4+2] reaction pathway with relatively more stable п-complexes (Ag-int1 and Cu-int1) as reference point.
With Ag-int1 as zero point, the barrier of silver(I) catalyzed stepwise mechanism is 33.3 kcal/mol and concerted mechanism 34 kcal/mol (Fig. 8a).
The barrier of copper-catalyzed [4+2] cycloaddition is very high with stepwise mechanism 39.3 kcal/mol and the concerted mechanism 43.3 kcal/mol (Fig. 8b). Obviously, [4+2] cyclization pathway is not favored both in silver(I) and copper(I) catalyzed IEDDA reaction.
Discussion
After formation of d10 metal (Ag+ or Cu+) coordinated four-member ring complex Ag-int3 or Cu-int3, we also evaluated the ring expansion and contraction way (Fig. 9). The rate determine barrier (RDB) of ring expansion is 27.5 kcal/mol and this process is energetically not favored compared with path a (RDB 20.3 kcal/mol). However, after formation of 1,2-NCOT, ring contraction to release N2 is energetically unfavored with barrier as high as 38.2 kcal/mol which means 1,2-NCOT is dynamic stable11,36,37,38. The barrier of formation 1,2-NCOT is just between our proposed SMC mechanism (20.8 kcal/mol) and stepwise [4+2] mechanism(33.3 kcal/mol). If the silylium ion transfer step was inhibited, 1,2-NCOT may be generated. Therefore, our calculation implies a very concise way to synthesis 1,2-NCOT.
Rawal et al. also declared that the electron-rich ethoxy alkynes 2c and ynamide 2d (Fig. 1b) cannot react with phthalazine 1a under the same condition as siloxy alkyne 2a. According our proposed SMC mechanism, both alkyenes 2c and 2d have no silicane groups and formation of ketene species was inhibited. For ethoxy alkyne 2c may possibility go through carbocation transfer to form ketene species after formation of four-member ring M-int3 (Ag-int3, Cu-int3) similarities, we evaluated barrier of this process as high as 34.1 kcal/mol. For ynamide 2d, it’s evident that the protection group cannot form any ion. If we change protection group of ynamide 2d into silane, this SMC pathway maybe also available to nitrogen-substituted alkynes. Actually electron-rich ethoxy alkynes 2c and ynamide 2d should be more facile to react with phthalazines 1a if this d10 metal-catalyzed IEDDA proceed through [4+2] cycloaddition pathway. It’s a strong support that protection group TIPS also take effect in this reaction.
For silver(I) and copper(I) catalyzed IEDDA, the difference of the rate determined barrier is 9.9 kcal/mol for path b, 6.5 kcal/mol for path a, and 6.0 kcal/mol for [4+2] path. In each case the barrier for copper(I) is higher which is in accordance with experiment. We think this is partly due to relative smaller radius and higher positive charge density of copper(I) atom, which lead to a stronger coordination of the substrate. According to our calculation, formation of L-Cu+ is 10 kcal/mol more exothermic than formation L-Ag+(−33.0 kcal/mol vs −23.1 kcal/mol). Formation of the п-complex Cu-int1 is much more exothermic than Ag-int1(−30.3 kcal/mol vs −60.8 kcal/mol, Fig. 3a). п-complex Cu-int1 is more stable than Ag-int1 in solution, which lead to a relatively lower reference point of copper(I) catalyzed IEDDA. This is main cause of relatively higher barrier of copper(I) catalyzed IEDDA. In addition, copper(I) atom is more prone to be oxidized, NBO charge distribution shows that there was evident electron transfer from copper(I) to siloxy alkyne which may also lead to stronger coordinate of copper(I) to siloxy alkynes (NBO charge distribution shows that the electron changed from −0.164 to −0.271 in the C2 and 0.293 to 0.248 in the C3 when siloxy alkyne 2a coordinated with copper(I) to form Cu-int1, electron charge changed into −0.218 in C2 and 0.312 in C3 when siloxy alkyne 2a coordinated with silver(I) to form Ag-int1).
Lewis acid-catalyzed IEDDA meet limited success which often confine to modification of the two п-electron components (dienophile or diene) for a better LUMO-HOMO matching. Our calculation provides a remarkably different perspective to access IEDDA cycloaddition product which is difficult obtain through traditional [4+2] pathway. In this reaction, high affinity of d10 metal (Ag+ or Cu+) to alkynes and alkenes activate the [2+2] cycloaddition and transfer of TIPS+ respectively. The electron extraction competition between d10 metal and TIPS+ lead to final product. In most silylium ion-catalyzed reaction, high acidity silylium ion should be prepared before experiment or generated in situ which often need highly active reagent, this п-acid and hard acid in situ exchange scheme may enlighten in silylium ion chemistry. This mechanism also implies a concise way to synthesis 1,2-dinitrogen substituted cyclooctatetraene (1,2-NCOTs) which is quite difficult to obtain with current methods. Nitrogen-substituted alkynes such as ynamines and ynamides are inert to IEDDA reaction, our SMC pathway may overcome this difficulty by change protection group into silicane.
Methods
Model reaction
Phthalazine 1a and ethyl substituted siloxy alkyne 2a was choose as the model reactions. 2,2′-bipyridine (bpy) was choose as the ligand in both silver(I) and copper(I) catalyzed IEDDA reactions. AgOTf and CuOTf was choose as catalyst salt respectively (Fig. 3a). Dichloromethane (DCM) was choose as the solvent.
Computational details
Density function calculations (DFT) were performed with the Gaussian 16 program package39. All structural optimizations were performed with M062x density functional40 with effective core potential (ECP) def2-TZVP41 for silver and 6-31 G(d,p) basis set42 for copper and other atoms (Optimized geometries see Supplementary Data 1–63). Single point energy was calculated at B2PLYPd3(BJ)/def2tzvp level of theory43. Vibrational frequency calculations were carried out at same level of theory as geometries optimization to verify that the optimized geometries is an energy minimum or a transition state and to provide thermal corrections for Gibbs free energies and enthalpies at 298.15 k in 1 atm. The intrinsic reaction coordinate (IRC) calculation was conducted to connect the transition structures with the corresponding reactants and products44 (Supplementary Figs. 5–11). Solvation calculations were carried out with CPCM solvation model45 at same level of theory with dichloromethane (DCM, ε = 8.93) as solvent. All the geometries were optimized in the gas phase except transition state of desilication step and inverse process which is optimized in solvent for electrostatic effect and complex conformation of triisopropylsilyl (TIPS) group make it very hard to load these structures in the gas phase.
Functionals test
It is commonly accepted that M06 or M06-d3 describes better the features of transition metals. We also optimized the key geometries of SMC pathway with both M06 and M06-d3 functionals. The M06 and M06-d3 optimized geometries are very similar (Supplementary method A). We corrected single point energy of M06-d3 optimized geometries under B2PLYPd3(BJ)/def2tzvp level of theory. Although M062x and M06-d3 predict slightly different geometries, the final reaction profiles based on M062x and M06-d3 optimized geometries are quite similar (Supplementary Figs. 2 and 3).
Data availability
The authors declare that all the other data supporting the findings of this study are available within this paper, its Supplementary Information file and Supplementary Data 1–63.
References
Jiang, X. & Wang, R. Recent developments in catalytic asymmetric inverse-electron-demand Diels–Alder reaction. Chem. Rev. 113, 5515–5546 (2013).
Glinkerman, C. M. & Boger, D. L. Catalysis of heterocyclic azadiene cycloaddition reactions by solvent hydrogen bonding: concise total synthesis of methoxatin. J. Am. Chem. Soc. 138, 12408–12413 (2016).
Kessler, S. N. & Wegner, H. A. Lewis acid catalyzed inverse electron-demand Diels− Alder reaction of 1, 2-diazines. Org. Lett. 12, 4062–4065 (2010).
Png, Z. M., Zeng, H., Ye, Q. & Xu, J. Inverse-electron-demand Diels-Alder reactions: principles and applications. Chem. Asian J. 12, 2142–2159 (2017).
Duret, G., Le Fouler, V., Bisseret, P., Bizet, V. & Blanchard, N. Diels-Alder and formal Diels-Alder cycloaddition reactions of ynamines and ynamides. Eur. J. Org. Chem. 2017, 6816–6830 (2017).
Duret, G. et al. Inverse electron-demand [4+2]-cycloadditions of ynamides: access to novel pyridine scaffolds. Org. Lett. 18, 1610–1613 (2016).
Smith, M. W. & Baran, P. S. As simple as 2+2. Science 349, 925–926 (2015).
Hoyt, J. M., Schmidt, V. A., Tondreau, A. M. & Chirik, P. J. ORGANIC CHEMISTRY iron-catalyzed intermolecular 2+2 cycloadditions of unactivated alkenes. Science 349, 960–963 (2015).
Bender, C. O., Dolman, D., Foesier, J. C., Lawson, S. L. & Preuss, K. E. The differing mechanisms of photo-formation of 7-cyanobenzocyclooctatetraene from 7- and 6-cyano-2,3-benzobicyclo[4.2.0]octa-2,4,7-triene. Can. J. Chem. 81, 37–44 (2003).
Deslongchamps, G. & Deslongchamps, P. Bent Bonds (tau) and the antiperiplanar hypothesis-the chemistry of cyclooctatetraene and other C8H8 isomers. J. Org. Chem. 83, 5751–5755 (2018).
Huang, Z., Zhang, W. X. & Xi, Z. Lewis acid-promoted ring-contraction of 2,4,6,8-tetrasubstituted 1,5-diazacyclooctatetraenes to 2,4,6-trisubstituted pyridines. Org. Lett. 20, 485–488 (2018).
Oishi, E. et al. Condensed pyridazines .8. Reaction of diazolopyridazines with ynamine - formation of benzodiazoles and diazolodiazocines. Chem. Pharm. Bull. 39, 1713–1718 (1991).
Iwamoto, K., Suzuki, S., Oishi, E., Miyashita, A. & Higashino, T. Ring transformation of fused pyridazines .4. Reaction of halo-substituted fused pyridazines with ynamines. Heterocycles 43, 199–204 (1996).
Hoffmann, R. & Woodward, R. B. Conservation of orbital symmetry. Acc. Chem. Res. 1, 17–22 (1968).
Turkmen, Y. E., Montavon, T. J., Kozmin, S. A. & Rawal, V. H. Silver-catalyzed formal inverse electron-demand Diels-Alder reaction of 1,2-diazines and siloxy alkynes. J. Am. Chem. Soc. 134, 9062–9065 (2012).
Sumaria, C. S., Turkmen, Y. E. & Rawal, V. H. Non-precious metals catalyze formal [4+2] cycloaddition reactions of 1,2-diazines and siloxyalkynes under ambient conditions. Org. Lett. 16, 3236–3239 (2014).
Avcı, Ö. N., Catak, S., Dereli, B., Aviyente, V. & Dedeoglu, B. Elucidation of the mechanism of silver-catalyzed inverse electron-demand Diels-Alder (IEDDA) reaction of 1,2-diazines and siloxy alkynes. ChemCatChem 12, 366–372 (2020).
Fang, G. & Bi, X. Silver-catalysed reactions of alkynes: recent advances. Chem. Soc. Rev. 44, 8124–8173 (2015).
Rode, N. D. et al. Synthesis of 2-Acylindoles via Ag- and Cu-Catalyzed anti-Michael Hydroamination of beta-(2-Aminophenyl)-alpha,beta-ynones: experimental Results and DFT Calculations. J. Org. Chem. 83, 6354–6362 (2018).
Royes, J. et al. Copper-catalyzed borylative ring closing C–C coupling toward spiro- and dispiroheterocycles. ACS Catal. 8, 2833–2838 (2018).
Douvris, C. & Ozerov, O. V. Hydrodefluorination of perfluoroalkyl groups using silylium-carborane catalysts. Science 321, 1188–1190 (2008).
Kim, K. C. et al. Crystallographic evidence for a free silylium ion. Science 297, 825–827 (2002).
Lambert, J. B., Zhang, S. H., Stern, C. L. & Huffman, J. C. Crystal-structure of a silyl cation with no coordination to anion and distant coordination to solvent. Science 260, 1917–1918 (1993).
Popov, S. et al. Teaching an old carbocation new tricks: Intermolecular C–H insertion reactions of vinyl cations. Science 361, 381–387 (2018).
Reed, C. A., Xie, Z. W., Bau, R. & Benesi, A. Closely approaching the silylium ion (R3SI+). Science 262, 402–404 (1993).
Shao, B., Bagdasarian, A. L., Popov, S. & Nelson, H. M. Arylation of hydrocarbons enabled by organosilicon reagents and weakly coordinating anions. Science 355, 1403–1407 (2017).
Wu, Q. et al. Characterization of hydrogen-substituted silylium ions in the condensed phase. Science 365, 168–172 (2019).
Lee, V. Y. Tricoordinate silyl cations (silylium ions). Russ. Chem. Rev. 88, 351–369 (2019)
Dias, H. V. R., Flores, J. A., Wu, J. & Kroll, P. Monomeric copper(I), silver(I), and gold(I) alkyne complexes and the coinage metal family group trends. J. Am. Chem. Soc. 131, 11249–11255 (2009).
Sweis, R. F., Schramm, M. P. & Kozmin, S. A. Silver-catalyzed 2+2 cycloadditions of siloxy alkynes. J. Am. Chem. Soc. 126, 7442–7443 (2004).
Hu, L. & Chen, H. Substrate-dependent two-state reactivity in iron-catalyzed alkene [2+2] cycloaddition reactions. J. Am. Chem. Soc. 139, 15564–15567 (2017).
Tao, J. Y., Fang, D. C. & Chass, G. A. Simplification through complexity: the role of Ni-complexes in catalysed diyne-cyclobutanone [4+2+2] cycloadditions, a comparative DFT study. Phys. Chem. Chem. Phys. 14, 6937–6945 (2012).
Mueller, T. In Functional Molecular Silicon Compounds I: Regular Oxidation States Vol. 155 Structure and Bonding (ed. Scheschkewitz, D.) 107–162 (Springer, 2014).
Yang, Y. F., Liang, Y., Liu, F. & Houk, K. N. Diels-Alder reactivities of benzene, pyridine, and di-, tri-, and tetrazines: the roles of geometrical distortions and orbital interactions. J. Am. Chem. Soc. 138, 1660–1667 (2016).
Yang, Y. F., Yu, P. & Houk, K. N. Computational exploration of concerted and zwitterionic mechanisms of Diels-Alder reactions between 1,2,3-triazines and enamines and acceleration by hydrogen-bonding solvents. J. Am. Chem. Soc. 139, 18213–18221 (2017).
Couchman, S. A., Thompson, T. K., Wilson, D. J., Dutton, J. L. & Martin, C. D. Investigating the ring expansion reaction of pentaphenylborole and an azide. Chem. Commun. 50, 11724–11726 (2014).
Eisch, J. J., Liu, W., Zhu, L. & Rheingold, A. L. Facile rearrangement of the 6,11-diphenyldibenzo[b,f][1,4]diazocine skeleton into a substituted 2-(2-aminophenyl)-1,3-diphenylisoindole via anomalous carbolithiation or hydrolithiation: corroboration of operative SET processes. Eur. J. Org. Chem. 2015, 7384–7394 (2015).
Zhang, S., Zhang, W. X. & Xi, Z. Semibullvalene and diazasemibullvalene: recent advances in the synthesis, reaction chemistry, and synthetic applications. Acc. Chem. Res. 48, 1823–1831 (2015).
Gaussian 16, Revision B.01, Frisch, M. J. et al. Gaussian, Inc., Wallingford CT, 2016.
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. PCCP 7, 3297–3305 (2005).
Binkley, J. S., Pople, J. A. & Hehre, W. J. Self-consistent molecular orbital methods. 21. Small split-valence basis sets for first-row elements. J. Am. Chem. Soc. 102, 939–947 (1980).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Fukui, K. The path of chemical reactions - the IRC approach. Acc. Chem. Res. 14, 363–368 (1981).
Barone, V. & Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998).
Acknowledgements
Financial support from National Key Research and Development Program of China (No. 2016YFB0600301), National Natural Science Foundation of China (21673224, 21873096), Chinese Academy of Sciences (XDB17010200), and Dalian National Laboratory for Clean Energy (DNL180204) are acknowledged.
Author information
Authors and Affiliations
Contributions
H.-D.W. conceived, designed the study and performed the calculations. H.-J.F. supervised the project and contributed the design of the research and oversaw the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, HD., Fan, HJ. Silylium ion mediated 2+2 cycloaddition leads to 4+2 Diels-Alder reaction products. Commun Chem 3, 126 (2020). https://doi.org/10.1038/s42004-020-00373-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-020-00373-2
This article is cited by
-
Silylium ion migration dominated hydroamidation of siloxy-alkynes
Communications Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.