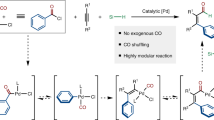
Abstract
Benzylic amines are valuable compounds with important applications in areas including pharmaceuticals and agrochemicals. The known procedures for their synthesis are limited by difficulties in functionalizing the parent aminomethyl groups. On the other hand, carbonylation reactions offer a potent method to introduce carbonyl groups and homologate carbon chains. However, carbonylative aminohomologation of aryl halides is challenging due to competing reactions and the need to balance multiple sequential steps. Here we report a palladium-catalyzed carbonylative aminohomologation reaction for the direct aminomethylation of aryl halides. The reaction proceeds via a tandem palladium-catalyzed formylation, followed by imine formation and formic acid-mediated reduction. Useful functional groups including chloride, bromide, ester, ketone, nitro, and cyano are compatible with this reaction. Both aryl iodides and bromides are suitable substrates and a wide range of synthetically useful amines are efficiently obtained in moderate to excellent yields.
Similar content being viewed by others
Introduction
Amines are important structural units that exist broadly in a large number of natural products, pharmaceuticals, and agrochemicals.1,2,3,4,5 For example, Vimpat is a medicine for the adjunctive treatment of partial-onset seizures and diabetic neuropathic pain. Gleevec is an oral medicine used for chronic myelogenous leukemia, acute lymphocytic leukemia, and certain types of gastrointestinal stromal tumors.
Hence, the construction of C–N bonds has attracted great interest from organic chemists.6,7,8,9,10,11 The traditional alkylation of ammonia usually produces a mixture of primary, secondary, and tertiary amines. Thus, the most widely used method for the preparation of amines is the reductive amination of aldehydes and ketones in the presence of a reducing agent. This procedure proceeds in one pot and avoids the preformation and isolation of imines, thus providing a convenient method for the synthesis of primary, secondary, and tertiary amines. Various reductants have been widely used as the hydrogen source.12,13 The main drawback of this strategy is the stability and availability of the aldehydes and ketones, which need to be prepared in advance. The Mannich reaction with formaldehyde in the presence of nucleophiles is another alternative for the synthesis of secondary amines.14
From the point of view of step economy and substrate availability, the direct, one-step aminomethylation of aryl or heteroaryl halides is a more attractive strategy for secondary amine synthesis. In this regard, Molander, Tanaka, and Dumas have independently developed the transition metal-catalyzed Suzuki-Miyaura cross-coupling reaction of aminomethyltrifluoroborates with aryl halides.15,16,17,18 Recently, Molander and co-workers18 reported a Ni/photoredox dual catalyzed aminomethylation of aryl halides using α-silylamines. A procedure based on tributyl(iodomethyl)stannane reagent was achieved as well and has been successfully applied in the synthesis of HIV NNRTI Doravirine analogs.19 The cross-coupling of aryl halides and N-aryl amines to generate benzylic amines was also established.20 Using nickel-photoredox catalysis via α-amino radical intermediates, the transformation proceeds effectively. In addition, the dehydrogenative aminomethylations of activated aromatic compounds with amines and MeOH have also been realized by the catalysis of metal (Ru, Mn, Fe) pincer complex.21,22,23 Despite their great efficiency, there are still some drawbacks to these reactions, such as the need to pre-functionalize substrates and a narrow substrate scope. Thus, developing general aminomethylation of aryl halides with non-expensive and easily available starting materials is still desirable.
Transition metal-catalyzed carbonylation reactions have emerged as a powerful platform for the synthesis of carbonyl-containing compounds.24,25,26 The hydroaminomethylation reaction of olefins had been developed in 1950s by Reppe at BASF, and had been improved by Beller.27 However to the best of our knowledge, the direct carbonylation-based aminomethylation of aryl halides has not been reported. One of the main challenges to this approach might be the competing aminocarbonylation reaction to give amides. Alternatively, hydrosilylation-based catalytic reduction of amides for the synthesis of amine has also been developed.28 Recently, we have developed a series of palladium-catalyzed carbonylation reactions with formic acid as a green CO source.29,30 In addition, we reported a palladium-catalyzed reductive carbonylation of aryl halides for the synthesis of aromatic aldehydes with formic acid as both CO source and reductant. Since formic acid is also widely used as a useful hydrogen surrogate, we propose that the direct carbonylation-based aminomethylation could be realized from aryl halides and amines in the presence of formic acid.
Herein, we report a palladium-catalyzed carbonylative aminohomologation (CAH) reaction of aryl halides using gaseous CO as the carbon source and formic acid as the reductant. Control experiments show that the aminomethyl group results from the reduction of imines which are formed from the reaction of amines with the in situ generated aldehydes.
Results
Optimization
Initially, 4-chloro-iodobenzene 1i and cyclohexanamine 2b were selected as model substrates for the CAH reaction (Fig. 1). Unsurprisingly, when 4-chloro-iodobenzene 1i was treated with cyclohexanamine 2b under a CO (2 bar) atmosphere with catalytic Pd(OAc)2 and PPh3 but without the formic acid reductant, only aminocarbonylation product amide 5ib was obtained. However, when the reaction was performed in the presence of formic acid, the aminomethylated product 3ib was obtained in 23% yield. In the reaction mixture, no aminocarbonylation product 5ib could be detected, and a small amount of dehalogenated product 6i was observed (Table 1, entry 1).
Inspired by this delighting result, we investigated the influence of different phosphine ligands (Table 1, entries 2–5, see details in Supplementary Table 1). Surprisingly, electron-rich monodentate phosphine PCy3, which showed best reactivity for the formylation of aryl halides in our previous work,29 is ineffective for the CAH reaction and no amine product could be detected (amide 5ib was obtained in 21% yield) (Table 1, entry 2). Sterically bulky ligand BuPAd2 slightly improved the yield to 28% (Table 1, entry 3). To our delight, substituted triarylphosphine ligands showed good reactivities for the CAH reaction. P(o-tolyl)3 was found to be the optimal ligand and the desired product could be isolated in 59% yield with a small amount of formamide 4ib (Table 1, entry 4). Bidentate phosphine ligands such as dppp and Xantphos were also active for the CAH reaction but were less effective and decreased yields were obtained (Table 1, entry 5). Reducing the amount of formic acid did not improve the selectivity of 3ib and 4ib.
Subsequently, various palladium catalysts such as Pd2(dba)3, Pd(CF3COO)2, and [PdCl(cinnamyl)]2 were tested in this reaction. All the catalysts showed good catalytic activities but with less selectivity between 3ib and 4ib (see details in Supplementary Table 2). The ratio of ligand and catalyst play an important role in transition metal catalysis. When the ratio was reduced from 6:1 to 2:1, the yield of 3ib increased to 70%. It should be mentioned that increasing the loading of catalyst and ligand resulted in both lower yields and selectivities (Table 1, entries 8 and 9). Further screening of the solvents revealed that CH3CN is the optimal solvent for this reaction (Table 1, entries 10–13, see details in Supplementary Table 3). Notably, our attempt to use formic acid as both the CO source and reductant failed, as the reaction of amine with DCC (formic acid activator) or formic acid proceeded much faster.
Substrate scope
With the optimized conditions in hand (Table 1, entry 10), we investigated the generality of the CAH reaction. Firstly, as summarized in Fig. 2, a series of different amines were subjected to the standard conditions and the corresponding aminomethylated products were obtained in moderate to good yields. Cyclic aliphatic amines were well tolerated and delivered the corresponding products in good yields. The steric properties of the amine affected the yield and the selectivity of the reaction. Sterically bulky amines resulted in higher selectivity but with lower yield (Fig. 2, 3aa–3ad). Then, various substituted anilines were subjected to the reaction conditions. Both electron-donating groups (Fig. 2, 3ae–3aj) and electron-withdrawing groups (Fig. 2, 3ak–3ap) were well tolerated and gave the corresponding products in 60–86% yields. Generally, electron-rich anilines resulted in slightly higher yields than anilines with electron-withdrawing groups. This can be explained by the stronger nucleophilicity of anilines with electron-donating substituents. Notably, halogen substituents (F, Cl, and Br) were tolerated without cleavage of the C–X bonds, which offers a handle for further transformations (Fig. 2, 3al–3ao). Interestingly, when linear primary amines such as benzylamine, n-butylamine, and n-octylamine were used in this reaction, tertiary amines could be obtained in 70%, 46%, and 49% yields, respectively (Fig. 2, 3aaq–3aas). This likely proceeds via a double aminomethylation reaction since the in situ generated secondary amines are more nucleophilic than the primary amines. Subsequently, secondary amines were investigated for this reaction. Pyrrolidine, N-methylaniline and N-methylbenzylamine gave the desired products in 62%, 60%, and 57% yields, respectively (Fig. 2, 3at–3av).
Palladium-catalyzed carbonylative aminohomologation of aryl iodides. Variation of aryl iodides and amines under standard conditions. a Testing of primary amines with iodobenzene. b Testing of secondary amines with iodobenzene. c Synthesis of tertiary amines from iodobenzene and linear primary amines. d Testing of aryl iodides with aniline. Reaction conditions: aryl iodide (0.5 mmol), amine (0.75 mmol), Pd(OAc)2 (0.5 mol%), P(o-tolyl)3 (1.0 mol%), Et3N (1.0 mmol), HCOOH (2.0 mmol), CH3CN (2 mL), CO (2 bar), 110 °C, 18 h, isolated yields
Then we turned our attention to the range of compatible aryl halides. As highlighted in Fig. 2, a series of aryl and heteroaryl iodides were successfully applied to the CAH reaction with aniline as the coupling partner. A useful range of functional groups including ether, chloride, bromide, ester, ketone, nitro, and cyano are compatible with these reaction conditions. A series of functionalized secondary amines could be synthesized with good yields (Fig. 2, 3be–3pe). Fluoro- and chloro-substituted iodobenzenes gave the desired products in 71% and 69% yields (Fig. 2, 3ge and 3he). But 4-bromo-iodobenene gave a low yield owing to the competitive dehalogenation reaction (Fig. 2, 3ie). It should be noted that the nitro group, which is usually not compatible in HCO2H-based carbonylation since it can be reduced or further transformed, can also be tolerated in this reaction and provided the corresponding product in 57% yield (Fig. 2, 3le). Moreover, 1-iodonaphthalene and heteroaryl iodides were also subjected to the CAH reaction, and the corresponding products were conveniently generated in good yields (Fig. 2, 3ne–3np).
With a slight optimization of the reaction conditions (see details in Supplementary Table 4), the CAH reaction is suitable for aryl bromides as well (Fig. 3). The use of sterically bulky ligand BuPAd2 and stronger base DBU facilitates the carbonylation of aryl bromides. As summarized in Fig. 3, using Pd(OAc)2 as the catalyst, BuPAd2 as the ligand and DBU as the base, the CAH of aryl bromides proceeds successfully at 120 oC. A range of anilines and aryl bromides are suitable substrates for this reaction. Various substituted anilines react with bromobenzene and yield the corresponding products in moderate yields (Fig. 3, 3ae–3al). On the other hand, a range of aryl and heteroaryl bromides are also compatible, with the corresponding products obtained in moderate yields (Fig. 3, 3be–3pe). Generally, the yields of aryl bromides are slightly lower than that of aryl iodides.
Palladium-catalyzed aminohomologation of aryl bromides. Substrates testing of anilines and aryl bromides. a Testing of bromobenzene with anilines. b Testing of aryl bromides with aniline. Reaction conditions: aryl bromide (0.5 mmol), amine (0.75 mmol), Pd(OAc)2 (2.0 mol%), BuPAd2 (4.0 mol%), DBU (1 mmol), HCOOH (4.0 mmol), CH3CN (2 mL), CO (2 bar), 120 °C, 18 h, isolated yields
To gain a better understanding of the reaction pathway of the CAH reaction, a series of control experiments were conducted (Fig. 4). First, when 13C-labeled carbon monoxide is used in the reaction of iodobenzene and 4-tert-butyl-aniline, the corresponding 13C-labeled amine is obtained in 80% yield. This result demonstrated that the homologated carbon originates from the carbon monoxide. Considering that either the amide or imine are potentially intermediates in this reaction, we tested the reaction of an amide and an imine under the standard conditions. No reaction could be observed when amide was used under the standard conditions, while the imine provided the desired product in 85% yield. In addition, the reaction of benzaldehyde and aniline under the standard conditions produced the amine in 88% yield. These results indicate that the CAH reaction might proceed via the imine, which was formed from the reaction of aniline and the in situ generated aldehyde.
Mechanistic Studies. Control experiments to uncover reaction pathways. a Replacement of CO with 13CO demonstrates the incorporation of carbon monoxide. b Failed reduction of N-phenyl benzamide excludes it as an intermediate. c Successful reduction of imine suggests its intermediacy. d In situ imine generation and reduction to further clarify the reaction intermediate. Standard reaction conditions: Pd(OAc)2 (0.5 mol%), P(o-tolyl)3 (1.0 mol%), Et3N (1.0 mmol), HCOOH (2.0 mmol), CH3CN (2 mL), CO (2 bar), 110 °C, 18 h, isolated yields
Discussion
Based on present results and previous literature, a plausible mechanism is proposed in Fig. 5. Initially, the oxidative addition of aryl halide to the Pd(0) forms the aryl palladium complex 7, which is converted to the acyl palladium 8 after coordination and insertion of CO. Then, formic acid reacts with the acyl palladium 8 and produces the aryl aldehyde 10 via a decarboxylative elimination. Catalytic active Pd(0) is regenerated in this step and used for the next cycle. Subsequently, the aldehyde 10 reacts with the amine to generate an imine intermediate 11, which is further reduced to the terminal amine product 3 with formic acid as the reductant.
In summary, we have developed a convenient procedure for the direct aminomethylation of aryl halides via a palladium-catalyzed CAH reaction. Both aryl iodides and bromides are suitable substrates for this transformation. The reaction tolerates a broad range of functional groups, affording the corresponding amines in moderate to excellent yields. Mechanistic studies suggest that the reaction proceeds via a palladium-catalyzed reductive carbonylation of aryl halides to produce aryl aldehydes, followed by imine formation with the amine and then formic acid-mediated reduction of the imine. Notably, efforts to use formic acid as both carbon source and reductant failed owing to competing reaction pathways.
Methods
Synthesis and characterization
See Supplementary Methods (general information about chemicals and analytical methods, synthetic procedures, and 1H and 13C NMR data) and Supplementary Figures 1–56 (1H and 13C NMR spectra).
Optimization
see Supplementary Table 1 (Optimization of ligands for aryl iodides), Supplementary Table 2 (Optimization of palladium precursors for aryl iodides), Supplementary Table 3 (Optimization of solvents for aryl iodides), Supplementary Table 4 (Optimization of the base for aryl bromide).
General procedure
Pd(OAc)2 (0.5 mol%) and P(o-tolyl)3 (1 mol%) were transferred into an 15 mL tube which was filled with nitrogen. Acetonitrile (2.0 mL), triethylamine (1 mmol), cyclohexylamine (0.75 mmol) iodobenzene (0.5 mmol), and formic acid were added to the reaction tube. Then the tube was purged and charged with CO. The tube was sealed and the mixture was stirred at 110 °C for 18 h. After the reaction was completed, the reaction mixture was filtered and concentrated under vacuum. The crude product was purified by column chromatography on silica gel to afford the corresponding product. Important to mention, the tiny impurities signals between 1 and 3 ppm in 1H NMR spectrum is due to the stabilizer in EtOAc which we used for purification, and do not have influence on the yields of the target products. Additionally, in the case that secondary benzyl amines were produced, their reaction with formic acid to give the N-formyl products occurs (as shown Fig. 1, 4ib). There is around 5% of N-formyl product which is difficult to be removed. The existing of N-formyl product has been taking into consideration in the yields calculation.
Data availability
The data sets generated and analyzed during the current study are included in the Supplementary Information file and also available from the corresponding authors on request.
References
Carey, J. S., Laffan, D., Thomson, C. & Williams, M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006).
Choi, D., Stables, J. P. & Kohn, H. Synthesis and anticonvulsant activities of N-benzyl-2-acetamidopropionamide derivatives. J. Med. Chem. 39, 1907–1916 (1996).
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 1, 493–502 (2002).
Deininger, M., Buchdunger, E. & Druker, B. J. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105, 2640–2653 (2005).
Hili, R. & Yudin, A. K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006).
Cho, S. H., Kim, J. Y., Kwak, J. & Chang, S. Recent advances in the transition metal-catalyzed twofold oxidative C-H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 40, 5068–5083 (2011).
Shin, K., Kim, H. & Chang, S. Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: a journey for the mild and versatile C-H amination. Acc. Chem. Res. 48, 1040–1052 (2015).
Song, G., Wang, F. & Li, X. C-C, C-O and C-N bond formation via rhodium(III)-catalyzed oxidative C-H activation. Chem. Soc. Rev. 41, 3651–3678 (2012).
Bariwalab, J., & Van der Eycken, E. C-N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 42, 9283–9303 (2013).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Marín, M., Rama, R. J. & Nicasio, M. C. Ni-catalyzed amination reactions: an overview. Chem. Rec. 16, 1819–1832 (2016).
Chatterjee, I. & Oestreich, M. Brønsted acid-catalyzed transfer hydrogenation of imines and alkenes using cyclohexa-1,4-dienes as dihydrogen surrogates. Org. Lett. 18, 2463–2466 (2016).
Wakchaure, V. N. & List, B. Catalytic asymmetric reductive condensation of N-H imines: synthesis of C2-symmetric secondary amines. Angew. Chem., Int. Ed. 55, 15775–15778 (2016).
Le Gall, E., Decompte, A., Martens, T. & Troupel, M. An expedient three-component synthesis of tertiary benzylamines. Synthesis 249–254 (2010).
Raushel, J., Sandrock, D. L., Josyula, K. V., Pakyz, D. & Molander, G. A. Reinvestigation of aminomethyltrifluoroborates and their application in Suzuki-Miyaura cross-coupling reactions. J. Org. Chem. 76, 2762–2769 (2011).
Murai, N., Miyano, M., Yonaga, M. & Tanaka, K. One-pot primary aminomethylation of aryl and heteroaryl halides with sodium phthalimidomethyltrifluoroborate. Org. Lett. 14, 2818–2821 (2012).
Molander, G. A. & Shin, I. Potassium Boc-protected secondary aminomethyltrifluoroborates: synthesis and Suzuki-Miyaura cross-coupling reactions. Org. Lett. 14, 4458–4461 (2012).
Remeur, C., Kelly, C. B., Patel, N. R. & Molander, G. A. Aminomethylation of aryl halides using α-silylamines enabled by Ni/photoredox dual catalysis. ACS Catal. 7, 6065–6069 (2017).
ElMarrouni, A., Campbell, M., Perkins, J. J. & Converso, A. Development of asp2−sp3 Stille cross-coupling for rapid synthesis of HIV NNRTI doravirine analogues. Org. Lett. 19, 3071–3074 (2017).
Ahneman, D. T. & Doyle, A. G. C-H functionalization of amines with aryl halides by nickel-photoredox catalysis. Chem. Sci. 7, 7002–7006 (2016).
Mastalir, M., Pittenauer, E., Allmaier, G. & Kirchner, K. Manganese-catalyzed aminomethylation of aromatic compounds with methanol as a sustainable C1 building block. J. Am. Chem. Soc. 139, 8812–8815 (2017).
Mondal, S., Samanta, S., Singsardar, M. & Hajra, A. Aminomethylation of imidazoheterocycles with morpholine. Org. Lett. 19, 3751–3754 (2017).
Kim, S. & Hong, S. H. Ruthenium-catalyzed aminomethylation and methylation of phenol derivatives utilizing methanol as the C1 source. Adv. Synth. Catal. 359, 798–810 (2017).
Brennführer, A., Neumann, H. & Beller, M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem., Int. Ed. 48, 4114–4133 (2009).
Gabriele, B.; Mancuso, R. & Salerno, G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 6825–6839 (2012).
Wu, X.-F. Palladium-catalyzed carbonylative transformation of aryl chlorides and aryl tosylates. RSC Adv. 6, 83831–83837 (2016).
Seayad, A. et al. Internal olefins to linear amines. Science 297, 1676–1678 (2002).
Volkov, A., Tinnis, F., Slagbrand, T., Trillo, P. & Adolfsson, H. Chemoselective reduction of carboxamides. Chem. Soc. Rev. 45, 6685–6697 (2016).
Wu, F.-P., Peng, J.-B., Meng, L.-S., Qi, X. & Wu, X.-F. Palladium-catalyzed ligand-controlled selective synthesis of aldehydes and acids from aryl halides and formic acid. ChemCatChem 9, 3121–3124 (2017).
Wu, F.-P., Peng, J.-B., Fu, L.-Y., Qi, X. & Wu, X.-F. Direct palladium-catalyzed carbonylative transformation of allylic alcohols and related derivatives. Org. Lett. 19, 5474–5477 (2017).
Acknowledgements
The authors acknowledge financial support from NSFC (21472174, 21602201, 21602204, 21772177) and the Zhejiang Natural Science Fund for Distinguished Young Scholars (LR16B020002).
Author information
Authors and Affiliations
Contributions
X.-F.W. and J.-B.P. conceived and supervised the project. F.-P.W. and C.X. performed the experiments, analyzed and prepared the supporting information. X.Q. and J.Y. participated in discussions. X.W. and J.P. wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, JB., Wu, FP., Xu, C. et al. Direct synthesis of benzylic amines by palladium-catalyzed carbonylative aminohomologation of aryl halides. Commun Chem 1, 29 (2018). https://doi.org/10.1038/s42004-018-0029-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42004-018-0029-8
This article is cited by
-
Nickel-catalysed carbonylative homologation of aryl iodides
Communications Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.