Abstract
A voice box (larynx) is unique for tetrapods and plays functional roles in respiration, airway protection, and vocalization. However, in birds and other reptiles, the larynx fossil is extremely rare, and the evolution of this structure remains largely unknown. Here we report the fossil larynx found in non-avian dinosaurs from ankylosaur Pinacosaurus grangeri. The larynx of Pinacosaurus is composed of the cricoid and arytenoid like non-avian reptiles, but specialized with the firm and kinetic cricoid-arytenoid joint, prominent arytenoid process, long arytenoid, and enlarged cricoid, as a possible vocal modifier like birds rather than vocal source like non-avian reptiles. Although bird-unique vocal source (syrinx) have never been reported in non-avian dinosaurs, Pinacosaurus could have employed bird-like vocalization with the bird-like large, kinetic larynx. This oldest laryngeal fossil from the Cretaceous dinosaur provides the first step for understanding the vocal evolution in non-avian dinosaurs toward birds.
Similar content being viewed by others
Introduction
A hyolaryngeal apparatus (tongue and voice box) is a key evolutionary trait in tetrapods and is associated with their feeding, respiration, and vocalization1,2,3,4,5,6,7,8,9,10,11,12. Particularly, the larynx is an entrance to the tracheal passage and is involved in vocalization (e.g., sound communication). Among modern archosaurs, the hyolaryngeal apparatuses of crocodilians and birds differ both anatomically and functionally7,10. In crocodilians, the larynx produces sound as a vocal source7. In birds, the syrinx produces sound at the posterior end of the trachea, and increases vocal efficiency as a vocal source10,13,14,15, while the larynx functions as a part of the vocal tract16. Although hyoids are sometimes preserved as fossils and the evolution of the hyoid has been previously discussed17,18, no larynx has been reported in extinct non-avian reptiles and the evolution of this organ is largely unknown14. This is probably because the larynx is cartilaginous in all tetrapods except neognath birds. Preserved hyoids in non-avian dinosaurs are only ceratobranchials except in the theropod Carnotaurus, Microraptor, and Confuciusornis (basihyal and ceratobranchial 1)18,19 and some ankylosaurs (basihyal, and ceratohyal, and ceratobranchial 1 in Saichania chulsanensis and paraglossal, ceratobranchial 1, ceratobranchial 2, and epibranchial in Pinacosaurus grangeri)17,20. Pinacosaurus grangeri (IGM100/3186) has the best-preserved hyolaryngeal apparatus in non-avian dinosaurs. Here we examine the hyolaryngeal apparatus of Pinacosaurus (IGM100/3186) and provide the first description of the larynx of non-avian dinosaurs with its comparisons to modern reptiles and birds.
Results
Description
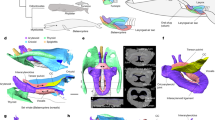
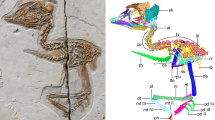
Pinacosaurus (IGM100/3186) preserves two laryngeal (cricoids and arytenoids) and one hyoid (ceratobranchials) elements (Fig. 1, Supplementary Note 1, Supplementary Figs. 1, 2, and 6). The symmetrical arrangement of these elements along the midline and the dorsal position of the arytenoid to the cricoid indicate that the hyolaryngeal apparatus is preserved in almost life position (Fig. 1a). Both cricoids (Fig. 1e, h) are complete but remain unfused at the midline, while those in the ankylosaurid Saichania are fused into a single element20. This probably has to do with the somatic immaturity of IGM100/318617. The cricoids are the largest bones of the hyolaryngeal apparatus. They are at least 64.7 mm wide and 65% of the mandible width. The cricoid is pointed anteriorly and widely expands posteriorly, with a concave posterior margin, forming an arrow-head shape in dorsal view. The anterior tip of the cricoid projects beyond the arytenoids, which may be an attachment site for the glottal constrictor muscle like ostrich10. The lateral edge of the cricoid body curls dorsally to form the cricoid wing. A dorsomedially projecting thin lamina extends anteroposteriorly from the anterior apex of the cricoid body and ends medial to the cricoid wing. At the intersection of the cricoid lamina and wing, a shallow groove (~2 cm long) forms an articular facet for the arytenoid (Fig. 1c). The dorsal surface above the groove has a rugose surface, indicating a cartilaginous pad for a joint with the arytenoid. A minute foramen lies near the posterior edge of the cricoid body on each side (Fig. 1a and Supplementary Fig. 1d), similar to two posterior notches in the cricoid of Alligator mississippiensis7 and the angular foramen on the cricoid in several birds (Supplementary Fig. 2) and chicken embryos21.
a Ventral view and b 3D reconstruction of skull, mandible, and hyolaryngeal apparatus in left oblique view. c Crico-aryteniod joint of right cricoid in medial view. d The joint of left arytenoid in dorsolateral view. e Arytenoid position in glottal opening and f glottal closing in anterior views. g Arytenoid position in glottal opening and h glottal closing in dorsal views. Abbreviations: afa, articular facet for arytenoid; afc, articular facet for cricoid; ap, arytenoid process; atr, atlas rib; caj, crico-arytenoid joint; lcb, left ceratobranchial; lcr, left cricoid; md, mandible; pm, premaxilla; pd, predentary; rar, right arytenoid; rcb, right ceratobranchial; rcr, right cricoid. Scale bars, 1 cm. Photograph by Michael D’Emic and edited by JY in a.
A pair of arytenoids lies dorsal to the cricoid (Fig. 1a, b, e–h). Those are anteroposteriorly long and bear short dorsolateral and long dorsomedial wings, which are J-shaped in cross-section (Fig. 1). The dorsomedial wing is thin, and its anterior third projects dorsally to form the arytenoid process as in turtles22 and birds10,23. The arytenoid process is an attachment site for m. dilator laryngis, which opens the glottis in crocodilians7,9 and birds10,11. The arytenoid process of Pinacosaurus is not as prominent as that of Saichania20 (Supplementary Fig. 3). This is probably due to its relative somatic immaturity. Like birds the arytenoid process ossifies late in ontogeny10. The dorsolateral wing is less developed than the dorsomedial wing. Furthermore, the lateral surface of the dorsolateral wing bears a ridge with a rugose surface that articulates with a corresponding rugose surface lying at the constriction of the above-mentioned cricoid groove (Fig. 1d). This forms the ridge-and-groove articulation between the cricoid and arytenoid. This unique morphology of the articular facet is not found in other reptiles including birds.
The paired ceratobranchials have proximal ends that lie medially along the midpoint axis of the body, while their distal ends splay laterally (Fig. 1a). The ceratobranchials were placed close to each other, and the distance between the proximal ends is about 2 mm. This is much smaller than the width of the cricoid. The ceratobranchial is slender, slightly curved ventrally, 36 mm long, about the half-length of the mandible, and ~70 % of the cricoid length (Fig. 1b, e–h).
Morphometrics
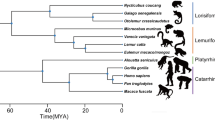
Arytenoids are short in larynges of non-dinosaurian reptiles and long in dinosaurs, such as Pinacosaurus and birds (Fig. 2a). A linear regression analysis shows that the arytenoid length is positively correlated with mandible width (Fig. 2a). However, a distinction in arytenoid size between a group of vocal source (i.e., reptiles) and that of non-vocal source but vocal modifier (i.e., birds) is also supported by student’s t-test of residuals of arytenoid length standardized by mandible width (t = −9.556, df = 87, p < 0.0001, Fig. 2b).
a Size of arytenoid (length) to mandible width in reptiles (n = 31) and birds (n = 58). (b) Box-plot of the standardized arytenoid length between birds and non-avian reptiles. c Size of cricoid (width) to mandible width in reptiles (n = 62) and birds (n = 90). d Box-plot of the standardized cricoid width among birds and non-avian reptiles. Linear regression lines showing the 95% confidence intervals of Ordinary Least Squares in a, c. Silhouettes from Phylopic (http://phylopic.org) by Andrew Farke, Dysalatornis, Michael Keesey, and Steven Traver.
Cricoid width is large in a gecko, vocal turtles, birds, and Pinacosaurus, and the cricoid width is positively correlated with mandible width (Fig. 2c). Birds differ in relative cricoid width from non-avian reptiles, which is statistically supported by Tukey HSD test of residuals of cricoid width standardized by mandible width (birds vs. crocodilians, p = 0.025; birds vs. squamates, vocal turtles, and quiet turtles, p < 0.0001, Fig. 2d).
Discussion
Fossil record and ossification of larynx in Archosauria
A fossilized larynx has not been reported in non-avian archosaurs and has been even rarely preserved in fossil birds. The oldest larynx in the fossil record was the cricoid of the anseriform Presbyornis (56–46 million years ago) from the Lower Eocene Green River Formation in Wyoming, USA24. Some hyolaryngeal elements of Pinacosaurus from the Cretaceous Djadokhta Formation and Saichania from the Cretaceous Baruungoyot Formation in Mongolia (84–72 million years ago), which were originally identified as the hyoids, paraglossal and ceratobranchial 117,20, are actually the larynx (cricoid and arytenoid) (Supplementary Fig. 3). Hence, this is the first report of the larynx in non-avian tetrapods during pre-Cenozoic eras (Fig. 3).
The ankylosaurs, Pinacosaurus (IGM100/3186) and Saichania (IGM100/151), share the ossification of cricoid and arytenoid (Supplementary Fig. 3) as in birds (Supplementary Fig. 2 and (ref. 25)). This indicates that the larynx is ossified in some non-avian dinosaurs. It still remains possible that the laryngeal ossification is ankylosaurid synapomorphy, or absent in other fossils simply due to poor preservation during fossilization or collection and preparation artifacts. In terms of development, the ossified larynx in the juvenile Pinacosaurus (IGM1000/13186) is similar to the early developmental condition in birds26.
Laryngeal morphology and vocalization of Pinacosaurus
The arytenoid process and firm cricoid-arytenoid joint in Pinacosaurus enable horizontal rotation of the arytenoid to open and close the glottis (Fig. 1e, h). This architecture could have controlled the laryngeal configuration and air pressure across the glottis. Only in birds does the arytenoid exhibit such a distinct arytenoid joint, in contrast to a connective tissue bridge as in other reptiles (Supplementary Figs. 4 and5). Furthermore, longer arytenoids in Pinacosaurus and birds than in non-dinosaurian reptiles (Fig. 2b) could result in a larger attachment area and a longer lever arm of the dilator muscle than the other reptiles, which enable them to open the glottis easily rather than close. In birds, the laryngeal glottis opens when it emits sounds produced by vibratory tissues in the syrinx27, while, in non-dinosaurian reptiles such as crocodilians and turtles9,28, the glottis is almost closed because it efficiently produces sound by the vibration of the vocal folds around the glottis dorsal or anterodorsal to arytenoids29. Therefore, the combination of the large arytenoid process, the firm cricoid-arytenoid joint, and the long arytenoids have likely allowed Pinacosaurus to open the glottis easily as in birds and may be used for avian-like airflow regulation, especially modifying a sound.
The larynges of dinosaurs (Pinacosaurus and birds) also show larger cricoids than non-dinosaurian reptiles (Fig. 2c). Similarly, vocal reptiles, such as gekkotans30,31 and some turtles (testudinids, trionychids, emydids, and batagurids)28,32,33, possess larger cricoids than non-vocal reptiles (Fig. 2c). The cricoid enlargement is common among the vocal groups, which can be explained by the flared end of the trachea as a part of the vocal tract and likely increases radiative efficiency34. Therefore, the larynx of Pinacosaurus may have been actively vocalized and associated with loud and explosive calls as in vocal reptiles and birds30,31,35,36.
In tetrapods, three different functions of larynx have been suggested: (1) airway protection (a barrier to foreign materials entering the trachea), (2) respiratory modulation (opening the glottis during respiration), and (3) acoustic communication (generation and modulation of sound)37,38. The large and kinetic larynx of Pinacosaurus, characterized by the large cricoid, firm cricoid-arytenoid joint, prominent arytenoid process, and long arytenoid, could be related to airflow regulation which functions in respiratory modulation and acoustic communication. Particularly, the four features of Pinacosaurus resemble that of birds, indicating that the kinetic larynx of Pinacosaurus is similar to birds such as parrots and passerines, which can largely change the configuration of the laryngeal cavity probably related to the complexity of vocalization11. Meanwhile, airway protection cannot explain these morphologies since it does not require a large and kinetic larynx for glottal closure. Therefore, the larynx of Pinacosaurus was specialized for opening the glottis and possibly a sound modifier with other vocal tracts such as the trachea and oral, esophageal, and pharyngeal cavities. Its vocalization might be related to courtship, parental call, predator defense, and territorial calls, as in modern archosaurs, crocodilians and birds35,36.
In birds, the power source for their vocalization is the anterior air sac system, which shrinks during exhalation39. Although osteological features for anterior air sac are absent in ankylosaurs and other ornithischian dinosaurs, their rib joint arrangement of the thoracic cavities are bird-like architecture40, indicating the presence of a dorsally immobilized lung like birds40 and anterior air sacs like saurischian dinosaurs (Saltasaurus41, Aerosteon, and also birds42) as a power source for sound production in non-avian dinosaurs. Although fossil evidence of the vocal source in non-avian dinosaurs has never been found so far, since Pinacosaurus is similar to birds in having a large, kinetic larynx and immobile lungs, this dinosaur likely possessed a non-laryngeal vocal source and enhanced their vocal activity and sound communication like modern birds.
Hyolaryngeal evolution of Archosauria
IGM100/3186 newly illustrates the evolution of diverse hyolaryngeal apparatus in Archosauria (Fig. 4). Ceratobranchial 2 is commonly present in non-archosaurian reptiles, and lost in Archosauria18. The hyolaryngeal apparatus of crocodilians is composed of four elements (arytenoid, cricoid, basihyal, and ceratobranchial 1), where all are cartilaginous except ceratobranchial 1. Pinacosaurus, an ornithischian dinosaur, retained the same hyolaryngeal elements as crocodilians. Yet, Pinacosaurus shows many shared characters with birds in the arrangement and morphology of the larynx (Fig. 4), such as, ossification of the larynx, an arytenoid process, a firm articular joint between the cricoid and arytenoid, long arytenoids, large cricoids, and a pointed tip of the cricoid (Fig. 1 and Supplementary Fig. 1). Paraglossal, procricoid, and epibranchial of birds are presumably retained in saurischian lineage (Fig. 4). As mentioned above, morphologies of the larynges indicate that Pinacosaurus did not use the larynx as a sound source like non-avian reptiles, but probably worked as a sound modifier like birds. We propose that bird-like vocalization have likely appeared in non-avian dinosaurs before the advent of Aves.
Ceratobranchial (blue), basihyal (white), paraglossal (orange), cricoid (purple), arytenoid (green), and procricoid (yellow). Colored lines indicate presence of the characters. A phylogenetic relationship of Testudines follows (ref. 43). A flesh-out reconstruction of Pinacosaurus with its hyolaryngeal apparatus (illustration by Tatsuya Shinmura). Silhouettes from Phylopic (http://phylopic.org) by Andrew Farke, Aline Ghilardi, Scott Hartman, Lukasiniho, Steven Traver, and Yan Wong.
Methods
Description of ankylosaur specimen
Ankylosaur dinosaur Pinacosaurus (IGM100/3186) is described as hyolaryngeal apparatus of fossil archosaurs. This specimen was discovered from Ukhaa Tolgod, Mongolia and its geological age is middle Campanian of the Late Cretaceous (17). The specimen is better prepared for observation and measurement after the previous publication in 2015. Computed Tomography and Laser scanning are conducted in AMNH to study its morphology. We used CT images to make a reconstruction of the larynx from open data at Morphobank (http://www.morphobank.org, ID: P2101).
Comparative study of larynx in modern and fossil skeletal specimens
For comparison, extant species of reptiles and birds (45 specimens of turtles, 14 of lizards, 4 of crocodilians, and 90 of birds) are examined qualitatively and quantitatively in American Museum of Natural History, New York, and National Museum of Nature and Science, Tokyo (Supplementary Information). We also studied the palaeognath bird skeletons, such as extant Nothura (AMNH10804), Nothoprocta (AMNH 6498, 6502), and extinct Dinornis (AMNH7301). Maximum transverse widths of cricoid and mandible, and maximum anteroposterior lengths of arytenoid are measured (Supplementary Fig. 1). For size normalization, all the measurements were normalized using the regression equation (Supplementary Data). For relative size comparisons, residuals of arytenoid length and cricoid width are obtained from mandible width, which are all log-transformed variables (Supplementary Data). All statistical analyses were performed using JMP Pro v.14. Linear regression analyses were conducted in two bi-plots: arytenoid length and mandible width, and cricoid and mandible widths. Student’s t test and Tukey HSD test were performed in the standardized arytenoid length and cricoid width.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The specimen of Pinacosaurus (IGM100/3186) is stored in the Institute of Paleontology in Ulaanbaatar, Mongolia. CT images for a reconstruction of the hyolarynx are openly available at Morphobank (http://www.morphobank.org, ID: P2101). Measurement data are available online at the Supplementary Information.
References
Reilly, S. M. & Lauder, G. V. The evolution of tetrapod feeding behavior: kinematic homologies in prey transport. Evolution 44, 1542–1557 (1990).
Iwasaki, S. Evolution of the structure and function of the vertebrate tongue. J. Anat. 201, 1–13 (2002).
Fitch, W. T. & Suthers, R. A. In Vertebrate Sound Production and Acoustic Communication (eds Suthers, R. A., Fitch, W. T., Fay, R. R., & Popper, A. N.) 1–18 (Springer, 2016).
Carroll, R. L. The Palaeozoic ancestry of salamanders, frogs and caecilians. Zool. J. Linn. Soc. 150, 1–140 (2007).
Schwenk, K. in Feeding: Form, Function and Evolution in Tetrapod Vertebrates (ed. Schwenk, K.) 175–291 (Academic Press, 2000).
Schwenk, K. & Rubega, M. In Physiological and ecological adaptations to feeding in vertebrates, (eds. Starck, M. & Wang, T.) 1–41 (Science Pub. Inc., 2005).
Schumacher, G. H. In Biology of the Reptilia, 4 (ed Gans, C.) 101–200 (Academic Press, 1973).
Reese, A. M. The laryngeal region of Alligator mississippiensis. Anat. Rec. 92, 273–277 (1945).
Riede, T., Li, Z., Tokuda, I. & Farmer, C. G. Functional morphology of the Alligator mississippiensis larynx with implications for vocal production. J. Exp. Biol. 218, 991–998 (2015).
McLelland, J. In Form and Function in Birds, 4 (eds King, A. S. & McLelland, J.) 69–103 (Academic Press, 1989).
Homberger, D. G. In The Biology of the Avian Respiratory System (ed Maina, J. N.) 27–97 (Springer, 2017).
Fitch, W. T. In Encyclopedia of Language & Linguistics (ed Brown, K.) 115–121 (Elsevier, 2006).
Clarke, J. A. et al. Fossil evidence of the avian vocal organ from the Mesozoic. Nature 538, 502–505 (2016).
Kingsley, E. P. et al. Identity and novelty in the avian syrinx. Proc. Natl Acad. Sci. USA 115, 10209–10217 (2018).
Riede, T., Thomson, S. L., Titze, I. R. & Goller, F. The evolution of the syrinx: an acoustic theory. PLoS Biol. 17, e2006507 (2019).
Nowicki, S. Vocal tract resonances in oscine bird sound production: evidence from birdsongs in a helium atmosphere. Nature 325, 53–55 (1987).
Hill, R. V. et al. A complex hyobranchial apparatus in a Cretaceous dinosaur and the antiquity of avian paraglossalia. Zool. J. Linn. Soc. 175, 892–909 (2015).
Li, Z. H., Zhou, Z. H. & Clarke, J. A. Convergent evolution of a mobile bony tongue in flighted dinosaurs and pterosaurs. PLoS One 13, e0198078 (2018).
Bonaparte, J. F., Novas, F. E. & Coria, R. A. Carnotaurus sastrei Bonaparte, the horned, lightly built carnosaur from the Middle Cretaceous of Patagonia. Contrib. in Sci. Nat. Hist. Mus. L. A. 416, 1–42 (1990).
Maryanska, T. Ankylosauridae (Dinosauria) from Mongolia. Palaeontol. Pol. 37, 85–151 (1977).
Mori, C. A comparative anatomical study on the laryngeal cartilages and laryngeal muscles of birds, and a developmental study on the larynx of the domestic fowl. Acta Med. 27, 2629–2678 (1957).
Siebenrock, F. Über den Kehlkopf und die Luftröhre der Schildkröten. Sitzungsberichte Der Kais. 108, 581–595 (1899).
Soley, J. T., Tivane, C. & Crole, M. R. Gross morphology and topographical relationships of the hyobranchial apparatus and laryngeal cartilages in the ostrich (Struthio camelus). Acta Zool. 96, 442–451 (2015).
Olson, S. L. & Feduccia, A. Presbyornis and the origin of the Anseriformes (Aves: Charadriomorphae). Smithson. Contrib. Zool. 323, 1–24 (1980).
Soley, J. T., Tivane, C. & Crole, M. R. A Gross morphology and topographical relationships of the hyobranchial apparatus and laryngeal cartilages in the ostrich (Struthio camelus). Acta Zool. 94, 442–451 (2015).
Hogg, D. A. Ossification of the laryngeal, tracheal and syringeal cartilages in the domestic fowl. J. Anat. 134, 57–71 (1982).
Gaunt, A. S., Stein, R. C. & Gaunt, S. L. Pressure and air flow during distress calls of the starling, Sturnus vulgaris (Aves; Passeriformes). J. Exp. Zool. 183, 241–261 (1973).
Sacchi, R., Galeotti, P., Fasola, M. & Gerzeli, G. Larynx morphology and sound production in three species of Testudinidae. J. Morphol. 261, 175–183 (2004).
Titze, I. R. The physics of small-amplitude oscillation of the vocal folds. J. Acoust. Soc. Am. 83, 1536–1552 (1988).
Russell, A. P., Hood, H. A. & Bauer, A. M. Laryngotracheal and cervical muscular anatomy in the genus Uroplatus (Gekkota: Gekkonidae) in relation to distress call emission. Afr. J. Herpetol. 63, 127–151 (2014).
Russell, A. P., Rittenhouse, D. R. & Bauer, A. M. Laryngotracheal morphology of Afro‐Madagascan Geckos: a comparative survey. J. Morphol. 245, 241–268 (2000).
Gans, C. & Maderson, P. F. Sound producing mechanisms in recent reptiles: review and comment. Am. Zool. 13, 1195–1203 (1973).
Galeotti, P., Sacchi, R., Fasola, M. & Ballasina, D. Do mounting vocalisations in tortoises have a communication function? A comparative analysis. Herpetol. J. 15, 61–71 (2005).
Fletcher, N. H. Bird song—a quantitative acoustic model. J. Theor. Biol. 135, 455–481 (1988).
Vergne, A. L., Pritz, M. B. & Mathevon, N. Acoustic communication in crocodilians: from behaviour to brain. Biol. Rev. 84, 391–411 (2009).
Marler, P. R. & Slabbekoorn, H. Nature’s music: The science of birdsong (Academic Press, San Diego, USA, 2004).
White, S. S. In Sisson and Grossman’s The Anatomy of the Domestic Animals. 2 (ed Getty, R.) 1891–1897 (Saunders, Philadelphia, USA 975).
Kirchner, J. A. The vertebrate larynx: adaptations and aberrations. Laryngoscope 103, 1197–1201 (1993).
Mackelprang, R. & Goller, F. Ventilation patterns of the songbird lung/air sac system during different behaviors. J. Exp. Biol. 216, 3611–3619 (2013).
Brocklehurst, R. J., Schachner, E. R. & Sellers, W. I. Vertebral morphometrics and lung structure in non-avian dinosaurs. R. Soc. Open Sci. 5, 180983 (2018).
Cerda, I. A., Salgado, L. & Powell, J. E. Extreme postcranial pneumaticity in sauropod dinosaurs from South America. Paläontol. Z. 86, 441–449 (2012).
Sereno, P. C. et al. Evidence for avian intrathoracic air sacs in a new predatory dinosaur from Argentina. PLoS One 3, e3303 (2008).
Chiari, Y., Cahais, V., Galtier, N. & Delsuc, F. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria). BMC Biol. 10, 65 (2012).
Acknowledgements
IGM and AMNH provided support for fieldwork. We thank C. Merrill, S. Goldberg, M. D’Emic, and A. Watanabe for technical support and C. Mehling, D. Kizirian, P. Capainolo, Y. Iwami, and I. Nishiumi for collection access. We acknowledge T. Shinmura for a life reconstruction of Pinacosaurus. This research was funded by Jurassic Foundation and Japan Society of the Promotion for Science (JSPS) Overseas Challenge Program for Young Researchers and KAKENHI Grant to JY. Other funding came from the Newt and Callista Gingrich Fund and the Macaulay Endowment to MN.
Author information
Authors and Affiliations
Contributions
J.Y. conceived and directed the study. J.Y. collected data and J.Y., Y.K., and M.N. performed analysis. J.Y., Y.K., and M.N. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Kenneth Carpenter and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Katie Davis and Luke R. Grinham.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshida, J., Kobayashi, Y. & Norell, M.A. An ankylosaur larynx provides insights for bird-like vocalization in non-avian dinosaurs. Commun Biol 6, 152 (2023). https://doi.org/10.1038/s42003-023-04513-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-023-04513-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.