Abstract
Gata2 is a key transcription factor required to generate Haematopoietic Stem and Progenitor Cells (HSPCs) from haemogenic endothelium (HE); misexpression of Gata2 leads to haematopoietic disorders. Here we deleted a conserved enhancer (i4 enhancer) driving pan-endothelial expression of the zebrafish gata2a and showed that Gata2a is required for HE programming by regulating expression of runx1 and of the second Gata2 orthologue, gata2b. By 5 days, homozygous gata2aΔi4/Δi4 larvae showed normal numbers of HSPCs, a recovery mediated by Notch signalling driving gata2b and runx1 expression in HE. However, gata2aΔi4/Δi4 adults showed oedema, susceptibility to infections and marrow hypo-cellularity, consistent with bone marrow failure found in GATA2 deficiency syndromes. Thus, gata2a expression driven by the i4 enhancer is required for correct HE programming in embryos and maintenance of steady-state haematopoietic stem cell output in the adult. These enhancer mutants will be useful in exploring further the pathophysiology of GATA2-related deficiencies in vivo.
Similar content being viewed by others
Introduction
Haematopoietic stem cells (HSCs) are the source of all blood produced throughout the lifetime of an organism. They are capable of self-renewal and differentiation into progenitor cells that generate specialised blood cell types. DNA-binding transcription factors are fundamental players in the inception of the haematopoietic system as it develops in the embryo, but also play a crucial role in maintaining homeostasis of the haematopoietic system in the adult organism. They coordinate differentiation, proliferation and survival of haematopoietic cells and ensure their levels are appropriate at all times throughout life. Misexpression of key transcription factors may thus lead to a failure to produce HSCs or, alternatively, to haematopoietic disorders and eventually leukaemia. Therefore, understanding how transcription factors drive the haematopoietic process provides opportunities for intervention when haematopoiesis is dysregulated.
The development of blood occurs in distinct waves: primitive, pro-definitive and definitive, each of them characterised by the generation of blood progenitors in a specific location and restricted in time, where the definitive wave produces multi-lineage self-renewing HSCs1. The specification of HSCs initiates in cells with arterial characteristics2 and proceeds through an endothelial intermediate, termed the haemogenic endothelium (HE)3. In zebrafish and other vertebrates, expression of runx1 defines the bona fide HE population4,5. Haematopoietic stem and progenitor cells (HSPCs) emerge from the HE by endothelial-to-haematopoietic transition (EHT), both in zebrafish and in mice6,7,8. They arise between 28 and 48 h post fertilisation (hpf) from the HE in the ventral wall of the dorsal aorta (DA)9, the analogue of the mammalian aorta-gonad-mesonephros (AGM)10. After EHT, the HSCs enter the bloodstream through the posterior cardinal vein (PCV)9 to colonise the caudal haematopoietic tissue (CHT), the zebrafish equivalent of the mammalian foetal liver11. Afterwards the HSCs migrate again within the bloodstream to colonise the kidney marrow (WKM) and thymus9, the final niche for HSCs, equivalent to the bone marrow in mammals1.
Gata2 is a key haematopoietic transcription factor (TF) in development. In humans, GATA2 haploinsufficiency leads to blood disorders, including MonoMAC syndrome (Monocytopenia, Mycobacterium avium complex) and myelodysplastic syndrome (MDS)12,13. While its presentation is variable, MonoMAC syndrome patients always show cytopenias, ranging from mild to severe, and hypocellular bone marrow13,14. These patients are susceptible to mycobacterial and viral infections, and have a propensity to develop MDS and Acute Myeloid Leukaemia (AML), with a 75% prevalence and relatively early onset at age 2013.
Gata2 knockout mice are embryonic lethal and die by E10.515. Conditional Gata2 knockout under the control of the endothelial VE-cad promoter abolished the generation of intra-aortic clusters16, suggesting that Gata2 is required for HSPC formation. Further studies in the mouse revealed a decrease in HSC numbers in Gata2 heterozygous mutants, but also a dose-dependency of adult HSCs on Gata217.
Gata2 expression in the endothelium is regulated by an intronic enhancer element termed the +9.5 enhancer18,19. Deletion of this enhancer results in the loss of HSPC emergence from HE, leading to lethality by E1419. The same element is also mutated in 10% of all the MonoMAC syndrome patients12.
Because of a partial genome duplication during the evolution of teleost fish, numerous zebrafish genes exist in the form of two paralogues, including gata220. This provides an opportunity to separately identify the temporally distinct contributions made by each Gata2 orthologue. Gata2a and gata2b are only 57% identical and are thought to have undergone evolutionary sub-functionalisation from the ancestral vertebrate Gata2 gene21,22. Gata2b is expressed in HE from 18hpf and is thought to regulate runx1 expression in HE21. Lineage tracing experiments showed that gata2b-expressing HE cells gave rise to HSCs in the adult21. Similar to the mouse Gata2, gata2b expression depends on Notch signalling and is a bona fide marker of HE, currently regarded as the functional ‘haematopoietic homologue’ of Gata2 in zebrafish21. By contrast, gata2a is expressed in all endothelial cells and in the developing central nervous system21,23. Homozygous gata2aum27 mutants showed arteriovenous shunts in the dorsal aorta at 48hpf24. However, gata2a is expressed at 11hpf in the haemangioblast population in the posterior lateral mesoderm (PLM) that gives rise to the arterial endothelial cells in the trunk25, well before gata2b is expressed in HE. This suggests that gata2a might play a role in endothelial and HE programming and thus help to elucidate an earlier role for Gata2 in HSC development.
Here we show that the gata2a locus contains a conserved enhancer in its 4th intron, corresponding to the described +9.5 enhancer in the mouse Gata2 locus18,19. Using CRISPR/Cas9 genome editing, we demonstrated that this region, termed the i4 enhancer, is required for endothelial-specific gata2a expression. Homozygous mutants (gata2aΔi4/Δi4 mutants) showed decreased expression of the HE-specific genes runx1 and gata2b. Thus, endothelial expression of gata2a, regulated by the i4 enhancer, is required for gata2b and runx1 expression in the HE. Strikingly, their expression recovers and by 48hpf, the expression of haematopoietic markers in gata2aΔi4/Δi4 mutants is indistinguishable from wild-type siblings. We have demonstrated that this recovery is mediated by an independent input from Notch signalling, sufficient to recover gata2b and runx1 expression in HE and thus HSPC emergence by 48hpf. We conclude that runx1 and gata2b are regulated by two different inputs, one Notch-independent input from Gata2a and a second from the Notch pathway, acting as a fail-safe mechanism for the initial specification of HSPCs in the absence of the input by Gata2a. Despite the early rescue, gata2aΔi4/Δi4 adults showed increased susceptibility to infections, oedema, a hypocellular WKM and neutropenia, a phenotype resembling key features of GATA2 deficiency syndromes in humans. We conclude that Gata2a is required for HE programming in the embryo and to maintain the steady-state haematopoietic output from adult HSPCs and that this function requires the activity of the i4 enhancer.
Results
Analysis of open chromatin regions in the gata2a locus
Because Gata2 genes are duplicated in zebrafish, we set out to unpick the different roles Gata2a and Gata2b play during HSC generation and homeostasis by identifying their regulatory regions. Analysis of sequence conservation revealed that one region within the fourth intron of the zebrafish gata2a locus was conserved in vertebrates, including mouse and human (Fig. 1a–c). This region, which we termed ‘i4 enhancer’, corresponds to the endothelial +9.5 Gata2 enhancer identified previously in the mouse18,19 and human26. Notably, the gata2b locus did not show broad conservation in non-coding regions (Supplementary Fig. 1a).
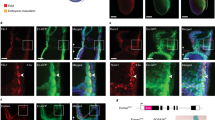
a Kdrl:GFP+ (green) and kdrl:GFP− (blue) cells were FACS-sorted from 26hpf embryos and used for preparation of ATAC-seq libraries. b The image of the mapped reads represents stacked means of two biological ATAC-seq replicates. Differential peak analysis identified four chromatin regions (blue shading) in the locus of gata2a that are significantly more open in the kdrl:GFP+ population (p < 0.0001). A region in the fourth intron (termed i4 enhancer) is conserved throughout vertebrates. Black and grey shading denotes regions of high conservation between the species analysed. c The highly conserved 150 bp region (red) contains putative transcription factor binding sites, mapped computationally. Light blue: Ets binding sites; purple: E-box binding sites; green: GATA binding sites; asterisks: conserved residues. d Widefield fluorescent image of a live Tg(gata2a-i4-1.1 kb:GFP) zebrafish embryo at 27hpf showing GFP fluorescence in the endothelial cells and in the heart (endocardium). e Higher magnification image of the trunk of the embryo from panel d. f–f″ Confocal images of a trunk fragment of a Tg(gata2a-i4-1.1 kb:GFP) embryo immunostained with anti-GFP antibody (f) and probed for gfp mRNA (f′) at 25hpf. f″ Merged images from panels f–f′ with Hoechst nuclear staining in blue, showing complete overlap of GFP protein and mRNA. g–g″ Confocal images of the dorsal aorta (DA) and posterior cardinal vein (PCV) of a Tg(gata2a-i4-1.1 kb:GFP) embryo immunostained with anti-GFP antibody (g) and probed for runx1 mRNA (g′) at 25hpf. See panel e for approximate position within the embryo. g″ Merged images from panels g–g’, also showing Hoechst nuclear staining in blue. h Counting of the runx1+ cells represented in panels g′–g″ in 25 embryos shows that >90% of runx1+ cells are also GFP+. N = 3. Error bars: ± SD. See also Supplementary Fig. 1.
To investigate whether the i4 element was a potentially active enhancer, we first performed ATAC-seq27 to identify open chromatin regions in endothelial cells (ECs) in zebrafish. We used a Tg(kdrl:GFP) transgenic line that expresses GFP in all endothelium28 and isolated the higher GFP-expressing ECs (kdrl:GFPhigh, termed kdrl:GFP+ for simplicity) as this fraction was enriched for endothelial markers compared to the kdrl:GFPlow fraction (Supplementary Fig. 1b, c). Principal Component Analysis on the ATAC-seq data from 26hpf kdrl:GFP+ cells (n = 2) and kdrl:GFP− cells (n = 4) revealed strong differences between the open chromatin regions in the two cell populations, further supported by a correlation analysis (Supplementary Fig. 1d–f). 78,026 peaks were found in common between replicates of the ATACseq in kdrl:GFP+ cells (Supplementary Fig. 1g). 44,025 peaks were differentially expressed between the kdrl:GFP+ and kdrl:GFP− fractions (Supplementary Fig. 1h). An analysis of known motifs present in the kdrl:GFP+ population revealed an enrichment for the ETS motif (Supplementary Fig. 1i). ETS factors are essential regulators of gene expression in endothelium29. In addition, we performed gene ontology (GO) term analysis on the peaks showing >3-fold enrichment or depletion in ECs (Supplementary Fig. 1j–l). As expected, non-ECs showed a broad range of GO terms whereas EC-enriched peaks were associated with terms like angiogenesis or blood vessel development (Supplementary Fig. 1k, l).
Differential peak analysis in the gata2a locus identified four differentially open sites within a 20 kb genomic region (Fig. 1b), including one peak in intron 4 corresponding to the predicted i4 enhancer. It contained a core 150 bp-long element that included several binding motifs for the GATA, E-box and Ets transcription factor families (Fig. 1b). Although the positioning of the E-box site relative to the adjacent GATA site differs in zebrafish and mammals (Fig. 1b, c), the necessary spacer distance of ~9 bp between the two sites30 was conserved. Thus, this site may be a target for TF complexes containing an E-box-binding factor and a GATA family TF.
Thus, the intronic enhancer (i4) identified in the zebrafish gata2a locus is accessible to transposase in endothelial cells and contains highly conserved binding sites for key haematopoietic transcription factors, suggesting that genetic regulation of gata2a expression in zebrafish HE is a conserved feature of vertebrate gata2 genes.
The gata2a-i4 enhancer drives GFP expression in endothelium
To investigate the activity of the gata2a-i4 enhancer in vivo, the conserved genomic 150 bp region (Fig. 1b, c), together with flanking ±500 bp (gata2a-i4-1.1 kb:GFP) or ±150 bp (gata2a-i4-450 bp:GFP) was cloned into a Tol2-based reporter E1b:GFP construct31 and used to generate stable transgenic lines (Supplementary Fig. 2). The earliest activity of the enhancer was observed at the 14-somite stage (14ss), when gfp mRNA was detected in the PLM (Supplementary Fig. 2a, b). After 22hpf, the reporter signal was pan-endothelial (Fig. 1d–e, Supplementary Fig. 2c–i). Around 27hpf, higher intensities of GFP fluorescence and correspondingly higher levels of gfp mRNA were visible in the floor of the DA (Fig. 1d–e, Supplementary Fig. 2e–h). While the GFP protein was still visible in the vasculature around 3dpf, it was likely carried over from earlier stages, since the gfp mRNA was not detectable any more (Supplementary Fig. 2i, j). We focussed our subsequent analysis on the gata2a-i4-1.1 kb:GFP transgenics as they showed stronger expression of the transgene. At 25hpf, the expression of GFP protein and gfp mRNA overlapped completely in the endothelial cells of the DA (Fig. 1f–f″). Overall, these data confirm that the i4 enhancer is active in vivo in endothelial cells at the correct time to regulate definitive haematopoiesis. The endothelial activity of the corresponding +9.5 enhancer was also observed in mouse embryos18, indicating functional conservation of the gata2a-i4 enhancer across vertebrates.
To further characterise the enhancer activity in vivo, Tg(gata2a-i4-1.1 kb:GFP) embryos were stained for gata2a mRNA and for GFP protein (Supplementary Fig. 2k–o). We found a large overlap between gata2a+ and GFP+ cells at 30hpf in the DA, with a small proportion of GFP+ cells that did not express gata2a mRNA (<5%, Supplementary Fig. 2o). This could suggest that some cells require activity of other endothelial enhancers to trigger transcription of gata2a or that gfp mRNA has a longer half-life than gata2a mRNA. Importantly, the GFP signal was absent in gata2a-expressing neural cells (Supplementary Fig. 2l–n), indicating that the i4 enhancer is specifically active in (haemogenic) endothelial cells.
Next we examined the expression of the HE marker runx14 in gata2a-i4-1.1 kb:GFP embryos at 25hpf. At this stage, over 90% of runx1+ cells were GFP+ (Fig. 1g–h). We conclude that the GFP expression under the gata2a-i4 enhancer marks the majority of the HE population.
Endothelial gata2a required gata2a-i4 enhancer activity
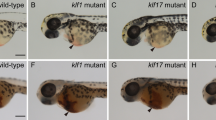
To investigate whether endothelial-specific expression of gata2a is required for definitive haematopoiesis, we deleted the conserved gata2a-i4 enhancer using CRISPR/Cas9 genome editing32. We generated a deletion mutant lacking 231 bp of the i4 enhancer (Supplementary Fig. 3a–c) and named it gata2aΔi4/Δi4. Homozygous gata2aΔi4/Δi4 mutants showed decreased levels of gata2a expression in endothelial cells when compared to wild-type embryos (Fig. 2a, b). By contrast, gata2a expression in the neural tube appeared unaffected in the gata2aΔi4/Δi4 mutants (Fig. 2a, b). At 28hpf, expression of the pan-endothelial marker kdrl was indistinguishable between wild-type and gata2aΔi4/Δi4 mutants (Fig. 2c, d). To verify these results, we crossed homozygous gata2aΔi4/Δi4 mutants to Tg(kdrl:GFP) transgenics and analysed vascular morphology. Gata2aΔi4/Δi4 embryos showed no gross vascular abnormalities at 48hpf as assessed by the expression of the Tg(kdrl:GFP) transgene (Fig. 2e, f).
a, b A significant majority of gata2aΔi4/Δi4 mutants have reduced levels of gata2a mRNA in the dorsal aorta (arrows) at 28hpf, compared to wild-type siblings, as detected with in situ hybridization. (Χ2 = 10.720, d.f. = 1, p < 0.01). The expression in the neural tube appears unaffected. c, d In situ hybridization for the endothelial marker kdrl at 28hpf reveals no difference between gata2aΔi4/Δi4 mutants and wild-type siblings. The dorsal aorta (arrows) appears unaffected. e, f Live images of the trunks of 48hpf Tg(kdrl:GFP) and Tg(kdrl:GFP); gata2aΔi4/Δi4 embryos show normal vascular morphology in the mutants. The endothelium of the dorsal aorta (arrows) appears normal in the gata2aΔi4/Δi4 embryos. g Kdrl:GFPhigh and kdrl:GFP− cells were sorted from non-mutant (WT, blue) and gata2aΔi4/Δi4 (red) embryos carrying the Tg(kdrl:GFP) transgene. h, i qRT-PCR on RNA isolated from the sorted kdrl:GFPhigh or kdrl:GFP- cells (panel g) shows decreased levels of gata2a mRNA in the endothelium of gata2aΔi4/Δi4 mutants at 23hpf (t = 20.026,d.f. = 5, p < 0.001) compared to wild-type. At 30hpf this difference is not statistically significant (t = 2.146, d.f. = 4, p = 0.098). There is no difference in gata2a mRNA levels in non-endothelial cells between wild-type and gata2aΔi4/Δi4 mutants (23hpf: t = 0.69, d.f. = 5, p > 0.5; 30hpf: t = 0.618, d.f. = 4, p > 0.5). N = 4 for gata2aΔi4/Δi4 at 23hpf, N = 3 for other samples. Note different scales of expression levels. ***p < 0.001. See also Supplementary Fig. 2.
Next, we isolated endothelial cells from Tg(kdrl:GFP) and Tg(kdrl:GFP); gata2aΔi4/Δi4 embryos by FACS (Fig. 2g) at 23hpf and 30hpf and confirmed by qRT-PCR that the endothelial markers kdrl, dld and dll4 were unaffected in gata2aΔi4/Δi4 embryos (Supplementary Fig. 3d–f). The arterial marker efnb2a33 was decreased at 23hpf in gata2aΔi4/Δi4 mutants but recovered by 30hpf (Supplementary Fig. 3g). In addition, gata2a was significantly decreased in the kdrl:GFP+ ECs in 23hpf gata2aΔi4/Δi4 embryos compared to wild types (Fig. 2h). At 30hpf this decrease was not statistically significant. This was likely due to a decrease in expression of gata2a that appears to occur in wild-type ECs during development, whereas gata2a expression in mutants remained low (Fig. 2h). Importantly, there was no difference in gata2a expression in the non-endothelial population (kdrl:GFP− cells) between wild-type and gata2aΔi4/Δi4 mutants at either 23 hpf or 30hpf (Fig. 2i). Altogether, these data suggest that genomic deletion of the gata2a-i4 enhancer is sufficient to reduce expression of gata2a specifically in endothelium.
Gata2a regulates runx1 and gata2b in haemogenic endothelium
To investigate a potential role of gata2a in HSC development, we compared the expression of runx1, the key marker of HE in zebrafish4, in wild-type and gata2aΔi4/Δi4 embryos. Quantitative in situ hybridization (ISH) analysis34 showed that runx1 expression was decreased in gata2aΔi4/Δi4 embryos at 24hpf (Supplementary Fig. 4a–c) and 28hpf compared to wild-type siblings (Fig. 3a–c). Further analysis in kdrl:GFP+ ECs showed that this decrease in runx1 expression was already detectable at 23hpf in gata2aΔi4/Δi4 mutants (Fig. 3d), at the onset of its expression in HE35. Thus, deletion of the gata2a-i4 enhancer results in impaired runx1 expression in the early stages of HE programming. This correlates well with decreased runx1 expression levels in +9.5−/− mouse AGM explants19, further supporting the critical evolutionary role of the intronic enhancer of Gata2 in HSC specification.
a, b In situ hybridization for runx1 expression in the HE of wild-type and gata2aΔi4/Δi4 embryos at 28hpf. c Quantification of the runx1 in situ hybridization signal from wild-type (blue), heterozygous gata2a+/Δi4 (het, yellow) and gata2aΔi4/Δi4 (red) siblings at 28hpf shows significant decrease in runx1 pixel intensity in the DA in the homozygous mutants compared to wild-type (µwt = 34.8, µmut = 25.3; F = 4.956, d.f. = 2, 58; ANOVA),**p < 0.01. n = 14, wild-type; n = 25, het; n = 23, gata2aΔi4/Δi4. Error bars: mean ± SD. d qRT-PCR on RNA isolated from the sorted kdrl:GFP+ cells shows decreased levels of runx1 mRNA in the endothelium of gata2aΔi4/Δi4 mutants at 23hpf (t = 2.585, d.f. = 5, p < 0.05) but not at 30hpf (t = 1.326, d.f. = 4, p > 0.2), compared to wild-type. N = 4 for gata2aΔi4/Δi4 at 23hpf, N = 3 for other samples. Note different scales of expression levels. *p < 0.05. e, f Gata2b expression in the HE of wild-type and gata2aΔi4/Δi4 embryos at 28hpf. g Quantification of the gata2b mRNA signal, detected by in situ hybridization, from wild-type (blue), heterozygous gata2a+/Δi4 (het; yellow) and gata2aΔi4/Δi4 (red) siblings at 28hpf shows significant decrease in gata2b pixel intensity in the DA in the homozygous mutants compared to wild-type (µwt = 39, µmut = 30.1; F = 5.05, d.f. = 2, 54; ANOVA), *p < 0.05. n = 22, wild-type; n = 24, het; n = 11, gata2aΔi4/Δi4. Error bars: mean ± SD. h qRT-PCR in sorted kdrl:GFP+ cells showed decreased levels of gata2b mRNA in the endothelium of gata2aΔi4/Δi4 mutants at 23hpf (t = 3.334, d.f. = 5, p < 0.05) but not at 30hpf (t = 0.373, d.f. = 4, p > 0.7), compared to wild-type. N = 4 for gata2aΔi4/Δi4 at 23hpf, N = 3 for other samples. *p < 0.05. See also Supplementary Figs. 3 and 4.
Next, we tested whether Gata2a could act upstream of gata2b by measuring its expression in gata2aΔi4/Δi4 embryos. Quantitation of the ISH signal showed that gata2b expression was decreased in gata2aΔi4/Δi4 embryos compared to wild-type siblings at 26hpf (Supplementary Fig. 4d) and 28hpf (Fig. 3e–g), but recovered to wild-type levels by 30hpf (Supplementary Fig. 4e). Accordingly, kdrl:GFP+; gata2aΔi4/Δi4 cells express significantly lower levels of gata2b mRNA than the wild-type kdrl:GFP+ endothelial population at 23hpf, but not at 30hpf (Fig. 3h). These data suggest that endothelial expression of gata2a is required upstream of gata2b and runx1 for the proper specification of HE, uncovering a previously unrecognized role for Gata2a in definitive haematopoiesis.
Recovery of embryonic HSC activity in gata2a Δi4/Δi4 mutants
The qPCR analysis in sorted kdrl:GFP+; gata2aΔi4/Δi4 and wild-type kdrl:GFP+ ECs (Fig. 3d) already suggested a recovery of runx1 expression from 30hpf. Of note, the kdrl:GFP+ population likely includes the kdrl+, runx1-expressing erythromyeloid progenitors (EMPs) located in the caudal region36. This region was not included in the quantification of ISH but cannot be separated by sorting for kdrl:GFP+ and could thus explain the discrepancy between image quantification and qRT-PCR. To further characterize the haematopoietic phenotype in the gata2aΔi4/Δi4 mutants, we tested whether expression of markers of haematopoietic activity in the embryo was affected from 48hpf onwards (Fig. 4).
a Representative image of runx1 expression in the trunk of a wild-type embryo at 48hpf showing runx1 mRNA in the dorsal aorta (arrow). b Quantification of the runx1 in situ hybridization signal in wild-type (blue) and gata2aΔi4/Δi4 mutants (red) siblings at 48hpf. There is no significant difference in runx1 pixel intensity in the DA between the homozygous mutants and wild-type (µwt = 33.1, µmut = 37.5, t = 1.410, d.f. = 44, p = 0.17. n = 19, wild-type; n = 27, gata2aΔi4/Δi4). Error bars: mean ± SD. c, d In situ hybridization for cmyb in the CHT. We detected no difference in expression between wild-type and gata2aΔi4/Δi4 siblings at 4dpf. (e–h) In situ hybridization (ventral view) for rag1 in the thymii, showing a slight decrease (relative to wild-type) in rag1 (red arrows) in approximately half of the homozygous mutant embryos at 4dpf. This effect is absent at 5dpf. (i, j) Maximum projections of itga2b:GFP transgenic embryos in the CHT at 5dpf in i wild-type and j gata2aΔi4/Δi4 siblings. k HSPC (itga2b:GFPlow) counts in the CHT of wild-type (n = 10) and gata2aΔi4/Δi4 mutants (n = 12) at 5dpf. No difference was detected between genotypes (μwt = 153.5; μmut = 145.5; p = 0.98, Mann-Whitney test). l Thrombocyte (itga2b:GFPhigh) counts in the CHT of wild-type (n = 10) and gata2aΔi4/Δi4 mutants (n = 12) at 5dpf. No difference was detected between genotypes (μwt = 13; μmut = 13; p = 0.71, Mann-Whitney test). Error bars: median ± SD.
At 48hpf, the expression of runx1 in the DA showed no significant difference between gata2aΔi4/Δi4 mutants and wild-type controls (Fig. 4a, b). These data suggest that the decrease of runx1 expression at early stages of HE programming in gata2aΔi4/Δi4 mutants is transient and recovers by 2dpf. Indeed, analysis of the HSPC marker cmyb11 in the CHT at 4dpf showed no differences between gata2aΔi4/Δi4 and wild-type larvae (Fig. 4c, d). Expression of the T-cell progenitor marker rag1 in the thymus37 showed that around half of the gata2aΔi4/Δi4 larvae had reduced rag1 expression at 4dpf compared to wild-type (Fig. 4e, f). This effect was absent at 5dpf (Fig. 4g, h), suggesting that HSPC activity was normal in gata2aΔi4/Δi4 mutants from 4dpf onwards. Next, we crossed the gata2aΔi4/Δi4 mutants to Tg(itga2b:GFP) transgenics, where itga2b-GFPhigh and itga2b-GFPlow cells in the CHT mark thrombocytes and HSPCs, respectively9,38. Our analysis revealed no difference in itga2b-GFPlow HSPC or itga2b-GFPhigh thrombocyte numbers in the CHT region at 5dpf between wild-type and gata2aΔi4/Δi4 mutants (Fig.4i–l). Taken together, our data suggest that endothelial gata2a expression mediated by the i4 enhancer is required for the initial expression of gata2b and runx1 in the HE but largely dispensable after 2dpf.
Notch recovers haematopoiesis in gata2a Δi4/Δi4 mutants
The recovery of gata2b expression by 30hpf (Fig. 3h, Supplementary Fig. 4e) coincides temporally with the observed decrease in gata2a in wild-type endothelial cells (Fig. 2h). Thus, we reasoned that other regulators of gata2b might compensate for the lack of endothelial gata2a in gata2aΔi4/Δi4 mutants and thus lead to a recovery of the initial haematopoietic phenotype. Therefore, we investigated whether the loss of gata2b in gata2aΔi4/Δi4 background resulted in a more severe haematopoietic phenotype than observed in the gata2aΔi4/Δi4 mutants. For this, we injected gata2aΔi4/Δi4 and wild-type controls with a suboptimal amount (7.5 ng) of a gata2b morpholino oligonucleotide (MO)21. Quantitative ISH analysis confirmed that this amount of gata2b MO had no effect on runx1 expression at 32hpf (Fig. 5a, b). As expected, runx1 expression in gata2aΔi4/Δi4 embryos was significantly reduced compared to wild-type siblings (Fig. 5a, b). Gata2b knockdown in gata2aΔi4/Δi4 embryos further reduced runx1 expression (Fig. 5a, b). To test whether this stronger reduction of runx1 at 32hpf affected later stages of embryonic haematopoiesis, we assessed cmyb expression in the CHT at 4dpf (Fig. 5c). We scored cmyb expression levels as ‘wild-type’ or ‘reduced’ and found that the ‘reduced’ embryos were substantially overrepresented in the gata2aΔi4/Δi4 mutants injected with the gata2b MO, compared to wild-type fish and non-injected gata2aΔi4/Δi4 siblings (Fig. 5c).
a Expression of runx1 in HE at 32hpf in wild-type (wt), gata2b MO-injected (7.5 ng) wt embryos, gata2aΔi4/Δi4 mutants and gata2b MO-injected (7.5 ng) gata2aΔi4/Δi4 mutants. b Quantification of the runx1 in situ hybridization (ISH) signal in wt, gata2b morphants, gata2aΔi4/Δi4 mutants and gata2aΔi4/Δi4 mutants injected with gata2b MO. runx1 expression is decreased in gata2aΔi4/Δi4 mutants (µwt = 41.9, µmut = 28.9; F = 44.641, d.f. = 3, 62.3; p < 0.001). Gata2b MO knockdown significantly decreases runx1 in the DA of gata2aΔi4/Δi4mutants (µmut = 28.9, µmut+MO = 17.2), but not wt embryos at 32hpf (µwt = 41.9, µMO = 40.1; p = 0.89, p = 0.89, Games-Howell post-hoc test, Welch’s ANOVA). n = 27, wt; n = 27, gata2aΔi4/Δi4; n = 33, wt + gata2b MO; n = 32, gata2aΔi4/Δi4 + gata2b MO. c Scoring cmyb expression at 4dpf in wt, gata2aΔi4/Δi4 mutants and gata2aΔi4/Δi4 mutant embryos injected with gata2b MO as wt (blue) or reduced (red). Gata2b MO knockdown (7.5 ng) inhibits the haematopoietic recovery of gata2aΔi4/Δi4 mutants. (Χ2 = 18.784, d.f. = 2, p < 0.001). d Quantification of the runx1 ISH signal, from 28hpf wt embryos (blue), gata2aΔi4/Δi4 mutants (red) and their siblings injected with a gata2a-i4-450bp:gata2b construct (shaded blue and red). Ectopic expression of gata2b increases runx1 expression in the HE of wt embryos (µwt = 38.8, µwt+gata2b = 53.4; p < 0.01) and rescues runx1 expression in the DA of gata2aΔi4/Δi4 mutants to wt levels (µmut = 17.9, µmut+gata2b = 33.2; p < 0.001; µwt = 38.8, µmut+gata2b = 33.2; p = 0.31, Tukey HSD post-hoc test). n = 25, wt; n = 33, gata2aΔi4/Δi4;n = 18, wt + gata2a-i4-450bp:gata2b construct; n = 17, gata2aΔi4/Δi4 + gata2a-i4-450bp:gata2b construct. e Quantification of the runx1 ISH signal at 36hpf in embryos treated with a suboptimal dose (25 μM) of the Notch inhibitor DAPM. 25 μM DAPM showed no effect on runx1 expression in wt compared to DMSO-treated embryos (µDMSO = 40.5, µDAPM = 38; p = 0.735, Tukey HSD post-hoc test). DMSO-treated gata2aΔi4/Δi4 mutants show a decrease in runx1 expression (µDMSO = 40.5, µmut+DMSO = 31.5; F = 25.774, d.f. = 3, 91; ANOVA). DAPM treatment significantly reduced runx1 expression in the DA gata2aΔi4/Δi4 mutants (µmut+DMSO = 31.5, µmut+DAPM = 19.4). n = 27, wt +DMSO; n = 20, gata2aΔi4/Δi4 + DMSO; n = 30, wt + DAPM; n = 20, gata2aΔi4/Δi4 + DAPM. f Representative images of the average runx1 expression at 36hpf in wt and gata2aΔi4/Δi4 mutants treated with 25 μM DAPM. Error bars: mean ± SD. **p < 0.01; ***p < 0.001. See also Supplementary Fig. 5.
To verify whether Gata2b is required for definitive haematopoiesis downstream of Gata2a, we generated a frameshift truncating mutant for Gata2b and incrossed gata2aΔi4/+; gata2b+/− adults to investigate cmyb expression at 33hpf in their progeny. Gata2b−/− mutants showed a more severe decrease in cmyb expression than gata2aΔi4/Δi4mutants (Supplementary Fig. 4f–i). Double gata2b−/−; gata2aΔi4/Δi4 mutants showed no further reduction in cmyb expression compared to gata2b−/− mutants, suggesting that Gata2a was not sufficient to drive cmyb expression in HE in the absence of Gata2b (Supplementary Fig. 4f–i). Taken together, we conclude that Gata2b is regulated by Gata2a and is required for definitive haematopoiesis.
Next, we tested whether forced ectopic expression of gata2b was sufficient to speed up the haematopoietic recovery of gata2aΔi4/Δi4 embryos. Thus, we overexpressed gata2b under the control of the gata2a-i4-450bp enhancer in wild-type and gata2aΔi4/Δi4 mutant embryos and measured runx1 expression at 28hpf in the DA. Gata2aΔi4/Δi4 embryos showed a significant decrease in runx1 expression in comparison to wild-type (Fig. 5d). Ectopic expression of gata2b under the gata2a-i4 enhancer significantly increased runx1 expression in wild-type and mutants (Fig. 5d). Importantly, it was sufficient to bring the runx1 expression levels in the mutants up to the levels detected in uninjected wild-type embryos (Fig. 5d), demonstrating that gata2b alone was sufficient to drive runx1 expression and drive the haematopoietic recovery in gata2aΔi4/Δi4 mutants. Thus, gata2b can recover the definitive haematopoietic programme in the absence of endothelial gata2a.
Because the expression of gata2b is regulated by Notch signalling21, we investigated whether inhibition of Notch would also prevent the haematopoietic recovery of gata2aΔi4/Δi4 embryos. For this, we used the Notch inhibitor DAPM39, and titrated it down to a suboptimal dose (25 μM) that did not significantly affect runx1 expression at 30hpf in wild-type embryos (Supplementary Fig. 5a). This dose induced a small but measurable decrease in gata2b expression in DAPM-treated embryos while higher doses had a more robust effect (Supplementary Fig. 5b). Next, we treated wild-type and gata2aΔi4/Δi4 mutant embryos with DAPM and measured runx1 expression in the DA at 36hpf (Fig. 5e, f). Suboptimal DAPM treatment did not affect runx1 expression in wild-type embryos (Fig. 5e, f), but gata2aΔi4/Δi4 mutants showed lower runx1 levels and DAPM treatment further reduced runx1 expression (Fig. 5e, f). Treatment with 25 μM DAPM did not significantly affect gata2b expression at 36hpf in either wild-type or gata2aΔi4/Δi4 mutant embryos (Supplementary Fig. 5c). These experiments suggested that Notch signalling alone may be sufficient to rescue expression of HE markers in gata2aΔi4/Δi4 mutants. Indeed, ectopic activation of Notch signalling in endothelium using a fli1a-NICD:GFP construct40 led to increased runx1 and gata2b expression in wild-type embryos (Supplementary Fig. 5d, e). When overexpressed in gata2aΔi4/Δi4 mutants, fli1a-NICD:GFP rescued runx1 expression to near wild-type levels at 26hpf, whereas gata2b was increased beyond normal levels independently of the genotype (Supplementary Fig. 5f, g). Taken together, these experiments confirm that Notch activity regulates runx1 and gata2b in HE and is sufficient to drive haematopoietic recovery in gata2aΔi4/Δi4 mutants. Thus, we conclude that HE programming requires two independent inputs on runx1 and gata2b expression; one from Gata2a, driven in ECs by the i4 enhancer, and the other from Notch signalling, necessary and sufficient to drive HE programming even in the absence of gata2a.
Impaired haematopoiesis in adult gata2a Δi4/Δi4 mutants
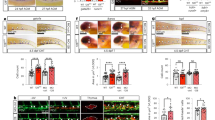
To investigate whether Gata2a plays a role in adult haematopoiesis, we first asked whether the gata2a-i4-1.1 kb:GFP reporter was active in haematopoietic cells in the adult. Whole kidney marrow (WKM) cells isolated from the transgenic fish showed that the i4 enhancer is active in haematopoietic cells previously defined by flow cytometry41 as progenitors, lymphoid + HSPC (containing the HSPCs) and myeloid cells (Supplementary Fig. 6a–c). Accordingly, single cell transcriptional profiling showed higher levels of gata2a in HSPCs, progenitors, neutrophils and thrombocytes (Supplementary Fig. 6d–f)42,43. Consistent with this notion, we observed a high incidence of infections and heart oedemas in gata2aΔi4/Δi4 adult fish, with over 25% suffering from one of these defects by 6 months of age, compared to <1% of wild-type fish (Fig. 6a–c). The heart oedemas and the infections are suggestive of lymphatic defects and immune deficiency as observed in human patients bearing genetic GATA2 haploinsufficiency syndromes such as MonoMAC syndrome13. Notably, around 10% of MonoMAC syndrome patients show mutations in the homologous enhancer region of GATA212,14.
a, b General morphology of zebrafish adults: a wild-type; b gata2aΔi4/Δi4 mutant showing skin infection (blue arrowhead) and pericardial oedema (yellow arrowhead). c Over 25% (n = 29/108) of gata2aΔi4/Δi4 mutants (red) catch infections or suffer from heart oedemas by 6 months. Only around 65% (n = 69/108) survive for more than 12 months without overt signs of infections. Fewer than 1% (n = 2/500) of wild-type fish (blue) exhibit such defects. The graph does not include deaths by other causes. d, e May-Grunwald/Wright-Giemsa staining in cytospins of haematopoietic cells isolated from the WKM of zebrafish adults: d wild-type; e gata2aΔi4/Δi4 mutant. Note the decrease in cell numbers. f Cell counts of haematopoietic cells isolated from WKM of wild-type (n = 14) and gata2aΔi4/Δi4 mutants (n = 8). The gata2aΔi4/Δi4 mutants show a ~2-fold decrease in haematopoietic cell numbers in the WKM (μwt = 4.37 × 105; μmut = 2.37 × 105, p = 0.0185, Mann-Whitney test). (g) Number of neutrophils isolated from WKM of wild-type (n = 14) and gata2aΔi4/Δi4 mutants (n = 7). The gata2aΔi4/Δi4 mutants show a ~2-fold decrease in neutrophil numbers in the WKM (μwt = 2.17 × 105; μmut = 1.03 × 105, p = 0.0269, Mann-Whitney test). Error bars: median cell number ± SD. h, i Kidney smears from 9 months post-fertilization adult animals were assessed. h Wild-type shows various stages of lineage differentiation. i WKM smear; 1 of 10 gata2aΔi4/Δi4 mutants showed the presence of excess blasts with very little erythroid differentiation (98% blasts, >200 cells assessed). Scalebars: 2 mm (a, b) and 10 μm (d, e, h, i). See also Supplementary Fig.6.
Next, we counted the total number of haematopoietic cells in wild-type and gata2aΔi4/Δi4 mutant WKM (Fig. 6d–f). To avoid any confounding effects in our analysis, we compared wild-type to gata2aΔi4/Δi4 mutants without overt signs of infection. The gata2aΔi4/Δi4 mutants showed a ~2-fold decrease in the total number of haematopoietic cells in the WKM (Fig. 6d–f). In addition, neutrophils were similarly reduced (Fig. 6g), another characteristic in common with MonoMAC syndrome patients14. Lastly, kidney marrow smears of ten 9-month old gata2aΔi4/Δi4 mutants were assessed. One of the ten mutants showed an excess of immature myeloid blast cells in the WKM (>98%) and only minor erythrocyte differentiation (Fig. 6h, i). The presence of excess blasts is usually an indication of AML in humans. Together these data strongly suggest that the i4 enhancer is a critical driver of gata2a expression in adult haematopoietic cells. The enhancer deletion in gata2aΔi4/Δi4 mutants leads to a hypocellular WKM and neutropenia, strongly suggestive of marrow failure, a hallmark of disease progression in Gata2 deficiency syndromes.
Discussion
The sub-functionalisation of the Gata2 paralogues in zebrafish provided an opportunity to unpick the different roles of Gata2 in the multi-step process of definitive haematopoiesis. Here we have investigated the conservation of the Gata2 +9.5 enhancer and identified a homologous region in intron 4 of the zebrafish gata2a locus (gata2a-i4) that is not present in the gata2b locus. The zebrafish gata2a-i4 enhancer, like the mouse enhancer18, is sufficient to drive pan-endothelial expression of GFP and necessary for endothelial expression of gata2a (Figs. 1 and 2). We traced the activity of the i4 enhancer back to the PLM, the source of precursors of endothelium and HSCs10. This degree of sequence and functional conservation of the i4 enhancer led us to hypothesize that Gata2a might play a role in definitive haematopoiesis. Indeed, homozygous deletion of the i4 enhancer (gata2aΔi4/Δi4) allowed us to uncover a previously unknown function of Gata2a in regulating the initial expression of runx1 and gata2b in HE. Although cmyb expression in HE was decreased in gata2aΔi4/Δi4 mutants, it was more severely reduced in gata2b−/− mutants, suggesting that Gata2b is more important for cmyb regulation than Gata2a. However, both Gata2 orthologues regulate gene expression in the HE before the first reported EHT events at 34hpf6.
Gata2 binds to the +9.5 enhancer to maintain its own expression in endothelial and haematopoietic cells26,44. In zebrafish, it is likely that Gata2a binds the GATA motifs in the i4 enhancer and loss of gata2a in the endothelium of gata2aΔi4/Δi4 mutants (Fig. 2) seems to support this view. Interestingly, we detected a small region in intron 4 of the gata2b locus that was not identified as a peak in our ATACseq experiment but is conserved in some fish species (Supplementary Fig. 1a) and thus could potentially represent a divergent gata2b intronic enhancer. We speculate that the positive autoregulation of Gata2 was likely retained by both gata2 orthologues in zebrafish, but this possibility remains to be investigated.
The gata2aΔi4/Δi4 mutants recovered from the early defects in HE programming and displayed normal expression levels of cmyb in the CHT at 4dpf and rag1 in the thymus at 5dpf, used as indicators of the definitive haematopoietic programme11. We hypothesized that this could be due to the presence of the two homologues of Gata2 in zebrafish20, despite Gata2a and Gata2b proteins being only 50% identical21. Indeed, forced expression of gata2b under the gata2a-i4 enhancer rescued DA expression of runx1 in the gata2aΔi4/Δi4 mutants to wild-type levels and suboptimal depletion of gata2b in the gata2aΔi4/Δi4 mutants resulted in more severe reduction in cmyb expression in the CHT by 4dpf (Fig. 4). In addition, we demonstrated that Notch signalling, a known regulator of gata2b expression21, is sufficient to rescue the initial HE programming defect induced by deletion of the gata2a-i4 enhancer. We propose a model in which gata2a acts upstream of runx1 and gata2b independently of Notch to initiate HE programming. The regulation of gata2b by Gata2a is transient, and the timing largely coincides with the natural decrease in endothelial expression of gata2a by 30hpf. After this stage, endothelial Notch signalling takes over the regulation of runx1 and gata2b expression, acting as a fail-safe mechanism that buffers against fluctuations in the system caused by loss of one or more of the initial inputs (in this case, Gata2a).
Despite the apparent haematopoietic recovery, we observed a high incidence of infections and oedema in gata2aΔi4/Δi4 adults, and a striking decrease in the number of haematopoietic cells in the WKM. The decrease in haematopoietic cells in particular is reminiscent of the loss of proliferative potential of haematopoietic Gata2+/− heterozygous cells in the mouse17,45. This raises the possibility that in zebrafish the gata2a and gata2b paralogues may function as two Gata2 ‘alleles’ that together regulate the haematopoietic output of the WKM. This will be addressed by comparing the adult phenotypes of gata2aΔi4/Δi4 and gata2b−/− mutants.
Taken together, our initial characterization of WKM shows that gata2aΔi4/Δi4 mutants present a phenotype consistent with Gata2 deficiency syndromes in humans brought about by GATA2 haploinsufficiency12,26. Strikingly, about 10% of all MonoMAC patients show mutations in the conserved +9.5 enhancer12,14, the corresponding regulatory element to the i4 enhancer. The i4 enhancer is active in the lymphoid + HSPC fraction that contains the HSC activity46, in the progenitor cells and in the myeloid fraction that contains eosinophils, previously identified as expressing high levels of a gata2a-GFP BAC transgenic reporter41. Thus, it is likely that gata2aΔi4/Δi4 adult fish show lineage-specific differentiation defects. Further characterization of the gata2aΔi4/Δi4 mutants will uncover which haematopoietic cells are most affected by the loss of i4 enhancer activity and how Gata2a regulates haematopoietic output, thus establishing a zebrafish animal model for human diseases linked to Gata2 haploinsufficiency.
Methods
Maintenance of zebrafish
Zebrafish (Danio rerio) were maintained in flowing system water at 28.5 °C, conductance 450–550 μS and pH 7.0 ± 0.5 as described47. Fish suffering from infections or heart oedemas were culled according to Schedule 1 of the Animals (Scientific Procedures) Act 1986. Eggs were collected by natural mating. Embryos were grown at 24–32 °C in E3 medium with methylene blue and staged according to morphological features corresponding to respective age in hours or days post-fertilization (hpf or dpf, respectively). Published strains used in this work were wild-type (wtKCL), Tg(−6.0itga2b:EGFP)la2 38,48 and Tg(kdrl:GFP)s843 28; animals were used at embryonic and larval stages and as adults (male and female) as specified in the figures. All animal experiments were approved by the relevant University of Oxford, University of Birmingham and Erasmus University ethics committees.
ATAC-seq
Tg(kdrl:GFP)s843 embryos were dissociated for FACS at 26-27hpf to collect kdrl+ and kdrl− cell populations (40,000–50,000 cells each). They were processed for ATAC library preparation using optimised standard protocol27. Briefly, after sorting into Hanks’ solution (1xHBSS, 0.25% BSA, 10 mM HEPES pH8), the cells were spun down at 500 g at 4 °C, washed with ice-cold PBS and resuspended in 50 µl cold Lysis Buffer (10 mM Tris-HCl, 10 mM NaCl, 3 mM MgCl2, 0.1% IGEPAL, pH 7.4). The nuclei were pelleted for 10 min. at 500 × g at 4 °C and resuspended in the TD Buffer with Tn5 Transposase (Illumina), scaling the amounts of reagents accordingly to the number of sorted cells. The transposition reaction lasted 30 min. at 37 °C. The DNA was purified with PCR Purification MinElute Kit (QIAGEN). In parallel, transposase-untreated genomic DNA from kdrl+ cells was purified with the DNeasy® Blood & Tissue Kit (QIAGEN). The samples were amplified with appropriate Customized Nextera primers27 in NEBNext High-Fidelity 2x PCR Master Mix (NEB). The libraries were purified with PCR Purification MinElute Kit (QIAGEN) and Agencourt AMPure XP beads (Beckmann Coulter). The quality of each library was verified using D1000 ScreenTape System (Agilent). Four biological replicas of the libraries were quantified with the KAPA Library Quantification Kit for Illumina® platforms (KAPA Biosystems). The libraries were pooled (including the Tn5-untreated control), diluted to 1 ng/µl and sequenced using 75 bp paired-end reads on Illumina HiSeq 4000 (Wellcome Trust Centre for Human Genetics, Oxford). Raw sequenced reads were checked for base qualities, trimmed where 20% of the bases were below quality score 20, and filtered to exclude adapters using Trimmomatic (Version 0.32) and mapped to Zv9 reference genome (comprising 14,612 genes)49 using BWA with default parameters. The results were visualised using UCSC Genome Browser (http://genome-euro.ucsc.edu/)50. The eight data sets were analysed with Principal Component Analysis (PCA) to identify outliers. Correlation among kdrl:GFP+ and kdrl:GFP− samples was assessed with a tree map. The peaks were called for each sample using the Tn5-untreated control as input. We identified the common peaks between replicates and then used DiffBind (EdgeR method) to identify differential peaks between kdrl:GFP+ and kdrl:GFP−samples (Supplementary Data 2). The threshold for differential peaks was p < 0.05.
Generation of transgenic and mutant zebrafish lines
Genomic regions containing the identified 150bp-long gata2a-i4 enhancer flanked by ±500 bp (i4–1.1 kb) or ±150 bp (i4–450bp) were amplified from wild-type zebrafish genomic DNA with NEB Phusion® polymerase (see Supplementary Table 1 for primer sequences) and cloned upstream of E1b minimal promoter and GFP into a Tol2 recombination vector (Addgene plasmid #3784551) with Gateway® cloning technology (Life Technologies™) following the manufacturer’s protocol. One-cell zebrafish embryos were injected with 1 nl of an injection mix, containing 50 pg gata2a-i4-E1b-GFP-Tol2 construct DNA + 30 pg tol2 transposase mRNA31. Transgenic founders (Tg(gata2a-i4-1.1 kb:GFP) and (gata2a-i4-450 bp:GFP)) were selected under a widefield fluorescent microscope and outbred to wild-type fish. Carriers of monoallelic insertions were detected by the Mendelian distribution of 50% fluorescent offspring coming from wild-type outcrosses. These transgenics were then inbred to homozygosity.
To generate the i4 deletion mutant, we identified potential sgRNA target sites flanking the 150 bp conserved region within intron 4 of the gata2a locus (see Fig. 1a, Supplementary Fig 3a). sgRNAs were designed with the CRISPR design tool (http:/crispr.mit.edu/, see Supplementary Table 1 for sequences) and prepared as described32. To reduce potential off-target effects of CRISPR/Cas9, we utilized the D10A ‘nickase’ version of Cas9 nuclease52,53, together with two pairs of sgRNAs flanking the enhancer (Supplementary Table 1, Supplementary Fig. 3a, b). We isolated two mutant alleles with deletions of 215 bp (Δ78–292) and 231 bp (Δ73–303) (Supplementary Fig. 3b). Both deletions included the highly conserved E-box, Ets and GATA transcription factor binding sites (Supplementary Fig. 3b). The Δ73–303 allele was selected for further experiments and named Δi4. Adult zebrafish were viable and fertile as heterozygous (gata2aΔi4/+) or homozygous (gata2aΔi4/Δi4). To unambiguously genotype wild types, heterozygotes and homozygous mutants, we designed a strategy consisting of two PCR primer pairs (Supplementary Fig. 3a, c). One primer pair flanked the whole region, producing a 600 bp wild-type band and 369 bp mutant band. In the second primer pair, one of the primers was designed to bind within the deleted region, only giving a 367 bp band in the presence of the wild-type allele (Supplementary Fig. 3c).
To generate the gata2b mutant we designed a CRISPR/Cas9 strategy for a frameshift truncating mutant in exon 3 deleting both zinc fingers. sgRNAs were designed as described above and guides were prepared according to Gagnon et al.54 with minor adjustments. Guide RNAs were generated using the Agilent SureGuide gRNA Synthesis Kit, Cat# 5190–7706. Cas9 protein (IDT) and guide were allowed to form ribonucleoprotein structures (RNPs) at RT and injected in 1 cell stage oocytes. 8 embryos were selected at 24 hpf and lysed for DNA isolation. Heteroduplex PCR analysis was performed to test guide functionality and the other embryos from the injection were allowed to grow up. To aid future genotyping we selected mutants by screening F1 for a PCR detectable integration or deletion in exon 3. Sequence verification showed that founder 3 had a 28 nt integration resulting in a frameshift truncating mutation leading to 3 new STOP codons in the third exon. To get rid of additional mutations caused by potential off-target effects, founder 3 was crossed to WT for at least 3 generations. All experiments were performed with offspring of founder 3.
Fluorescence-activated cell sorting (FACS)
Appoximately 100 embryos at the required stage were collected in Low Binding® SafeSeal® Microcentrifuge Tubes (Sorenson) and pre-homogenized by pipetting up and down in 500 µl Deyolking Buffer (116 mM NaCl, 2.9 mM KCl, 5 mM HEPES, 1 mM EDTA). They were spun down for 1 min. at 500 g and incubated for 15 min. at 30 °C in Trypsin + Collagenase Solution (1xHBSS, 0.05% Gibco® Trypsin + EDTA (Life Technologies™), 20 mg/ml collagenase (Sigma)). During that time, they were homogenized by pipetting up and down every 3 min. The lysis was stopped by adding 50 µl foetal bovine serum and 650 µl filter-sterilized Hanks’ solution (1xHBSS, 0.25% BSA, 10 mM HEPES pH8). The cells were rinsed with 1 ml Hanks’ solution and passed through a 40 µm cell strainer (Falcon®). They were resuspended in ~400 µl Hanks’ solution with 1:10,000 Hoechst 33258 (Molecular Probes®) and transferred to a 5 ml polystyrene round bottom tube for FACS sorting. The cells were sorted on FACSAria Fusion sorter by Kevin Clark (MRC WIMM FACS Facility). The gates of GFP (488–530) and DsRed (561–582) channels were set with reference to samples derived from non-transgenic embryos. The fluorescence readouts were compensated when necessary. For ATAC-seq library preparation, the cells were sorted into Hank’s solution. For RNA isolation, the cells were sorted directly into RLT Plus buffer (QIAGEN) + 1% β-mercaptoethanol and processed with the RNEasy® Micro Plus kit (QIAGEN), according to the accompanying protocol. The RNA was quantified and its quality assessed with the use of Agilent RNA 6000 Pico kit. All RNA samples were stored at −80 °C.
SYBR® Green qRT-PCR
3 µl of the cDNA diluted in H2O were used for technical triplicate qRT-PCR reactions of 20 µl containing the Fast SYBR® Green Master Mix (Thermo Fisher Scientific) and appropriate primer pair (see Supplementary Table 1). The reactions were run on 7500 Fast Real-Time PCR System (Applied Biosystems) and the results were analysed with the accompanying software. No-template controls were run on each plate for each primer pair. Each reaction was validated with the melt curve analysis. The baseline values were calculated automatically for each reaction. The threshold values were manually set to be equal for all the reactions run on one plate, within the linear phase of exponential amplification. The relative mRNA levels in each sample were calculated by subtracting the geometric mean of Ct values for housekeeping genes eef1a1l1 and ubc from the average Ct values of the technical triplicates for each gene of interest. This value (ΔCt) was then converted to a ratio relative to the housekeeping genes with the formula 2−ΔCt.
Fluidigm Biomark qRT-PCR
To quantify the differences in gata2a expression between wild-type and mutant ECs, we crossed homozygous gata2aΔi4/Δi4 mutants to Tg(kdrl:GFP) transgenics to generate Tg(kdrl:GFP); gata2aΔi4/Δi4 embryos. These fish, along with non-mutant Tg(kdrl:GFP), were used for FACS-mediated isolation of kdrl:GFP+ and kdrl:GFP− cells to quantitatively compare mRNA expression levels of gata2a in the endothelial and non-endothelial cells of wild-type and gata2aΔi4/Δi4 embryos, using the Fluidigm Biomark™ qRT-PCR platform. Briefly, 1 ng RNA from FACS-sorted cells was used for Specific Target Amplification in a 10 µl reaction with the following reagents: 5 µl 2xBuffer and 1.2 µl enzyme mix from SuperScript III One-Step Kit (Thermo Fisher Scientific), 0.1 µl SUPERase• In™ RNase Inhibitor (Ambion), 1.2 µl TE buffer (Invitrogen), 2.5 µl 0.2x TaqMan® assay mix (see Supplementary Table 2 for the details of TaqMan® assays). The reaction was incubated for 15 min. at 50 °C, for 2 min. at 95 °C and amplified for 20 cycles of 15 s at 95 °C/4 min. at 60 °C. The cDNA was diluted 1:5 in TE buffer and stored at −20 °C. Diluted cDNA was used for qRT-PCR according to the Fluidigm protocol for Gene Expression with the 48.48 IFC Using Standard TaqMan® Assays (Supplementary Table 2). Each sample was run in 3–4 biological replicates. The collected data were analysed with Fluidigm Real-Time PCR Analysis software (version 4.1.3). The baseline was automatically corrected using the built-in Linear Baseline Correction. The thresholds were manually adjusted for each gene to fall within the linear phase of exponential amplification, after which they were set to equal values for the housekeeping genes: rplp0, rpl13a, cops255, lsm12b56 and eef1a1l1. The relative mRNA levels for each sample were calculated by subtracting the geometric mean of Ct values for the housekeeping genes from the Ct value for each gene of interest. This value (ΔCt) was then converted to a ratio relative to the housekeeping genes with the formula 2−ΔCt. The ΔCt values were analysed with 2-tailed paired-samples t-tests with 95% confidence levels.
Flow cytometry and isolation of WKM haematopoietic cells
Single cell suspensions of WKM cells were prepared from adult zebrafish kidneys of the required genotypes as described57. Briefly, adult zebrafish were first euthanized in 0.5% tricaine in PBS and dissected to remove the kidney. WKM cells were recovered by vigorous pipetting in 0.5 ml PBS with 10% Foetal Calf Serum (PBS + 10%FCS), followed by filtration in a cell strainer (FALCON, ref 352235) pre-coated with PBS + 10%FCS. Strainers were rinsed with PBS + 10%FCS and the cells spun down (~300 g, 10 min at 4 °C) and ressuspended in 200–500 μl PBS + 10%FCS with Hoechst 33342 1:10000 (Hoechst 33342, H3570, ThermoScientific). Flow cytometry analysis was performed on a FACS Aria II (BD Biosciences) after exclusion of dead cells by uptake of Hoechst dye, as described41. WKM cell counts were performed on a PENTRA ES60 (Hariba Medical) following the manufacturer’s instructions. Note that the cell counter does not recognize the zebrafish nucleated erythrocytes, so these were excluded from this analysis. Cell counts for each genotype were analysed with 2-tailed paired-samples t-tests with 95% confidence levels, using a Mann-Whitney test for non-parametric distribution. The scatter plots were generated using GraphPad Prism 8.0 and show medians ± SD.
May-Grunwald and Wright-Giemsa staining
Cell staining with May-Grunwald (MG) stain (Sigma MG500) and Giemsa (GIEMSA STAIN, FLUKA 48900) was performed on haematopoietic cell samples. After cytospin, slides are allowed to air-dry and were stained for 5 min at room temperature with a 1:1 mix of MG:distilled water. Next, slides were drained and stained with a 1:9 dilution of Giemsa:distilled water solution for 30 min at room temperature. Excess solution was drained and removed by further washes in distilled water. Finally, the slides were air-dried and mounted in DPX (06522, Sigma) for imaging.
Whole-mount in situ hybridization and immunohistochemistry
Whole-mount in situ hybridization (ISH) was carried out as described previously58, using probes for kdrl, runx1, cmyb, gata2a, gata2b, rag14,37,59,60 and gfp (Supplementary Table 1). For conventional ISH embryos were processed, imaged and the ISH signal quantified as described34. Briefly, the pixel intensity values were assessed for normal distribution with a Q-Q plot and transformed when necessary. Mean values (µ) of each experimental group were analysed with 2-tailed independent-samples t-tests or with ANOVA with 95% confidence levels, testing for the equality of variances with a Levene’s or Brown-Forsythe test and applying the Welch correction when necessary. For ANOVA, differences between each two groups were assessed with either Tukey’s post-hoc test (for equal variances) or with Games-Howell test (for unequal variances). For all these analyses, the IBM® SPSS® Statistics (version 22) or GraphPad Prism 8.0 package were used.
For the analysis of cmyb expression in the CHT at 4dpf, the embryos scored as ‘high’ or ‘low’ were tested for equal distribution between morphants and uninjected controls or among wild-type, heterozygous and mutant genotypes with contingency Chi-squared tests, applying Continuity Correction for 2 × 2 tables, using IBM® SPSS® Statistics (version 22).
For fluorescent ISH (FISH) combined with immunohistochemistry, ISH was performed first following the general whole-mount in situ hybridisation protocol. The signal was developed with SIGMAFAST Fast Red TR/Naphthol, the embryos rinsed in phosphate-buffered saline with tween20 (PBT) and directly processed for immunohistochemistry. Embryos were blocked in blocking buffer (5% goat serum/0.3% Triton X-100 in PBT) for 1 h at RT before incubated with primary antibody against GFP (rabbit, 1:500, Molecular Probes), diluted in blocking buffer overnight at 4 °C. Secondary antibody raised in goat coupled to AlexaFluor488 (Invitrogen) was used in 1:500 dilutions for 3 h at RT. Hoechst 33342 was used as a nuclear counterstain.
Fluorescent images were taken on a Zeiss LSM880 confocal microscope using ×40 or ×63 oil immersion objectives. Images were processed using the ZEN software (Zeiss).
Fluorescence microscopy and cell counting
For widefield fluorescence microscopy, live embryos were anaesthetised with 160 μg/ml MS222 and mounted in 3% methylcellulose and imaged on a AxioLumar V.12 stereomicroscope (Zeiss) equipped with a Zeiss AxioCam MrM. To count itga2b-GFPhigh and itga2b-GFPlow cells in the CHT, Tg(itga2b:GFP;kdrl:mCherry); gata2aΔi4/+ animals were incrossed and grown in E3 medium supplemented with PTU to prevent pigment formation. At 5dpf, the larvae were anaesthetised with MS222 and the tail was cut and fixed for 1 h at room temperature in 4% PFA. Next, the tails were mounted on 35 mm glass bottomed dishes (MAtTEK) in 1% low melt agarose and imaged using a ×40 oil objective on an LSM880 confocal microscope (Zeiss). Cells in the CHT region were counted manually on Z-stacks as ‘itga2b:GFPlow’ (HSPCs) or ‘itga2b:GFPhigh’ (thrombocytes). Genomic DNA from the heads was extracted and used for genotyping as described above. Cell counts for each genotype were analysed with 2-tailed paired-samples t-tests with 95% confidence levels, using a Mann-Whitney test for non-parametric distribution. The graphs were generated using GraphPad Prism 8.3.0 and show medians ± SD.
Statistics and reproducibility
Data were analysed using either IBM® SPSS® Statistics (version 22) or GraphPad Prism software (v8.02). In situ quantification data was analysed with 2-tailed independent-samples t-tests or with ANOVA with 95% confidence levels, testing for the equality of variances with a Levene’s or Brown-Forsythe test and applying the Welch correction when necessary. For ANOVA, differences between each two groups were assessed with either Tukey’s post-hoc test (for equal variances) or with Games-Howell test (for unequal variances). Alternatively, data were analysed with an appropriate non-parametric test (Kruskall-Wallis) followed by Dunn’s multiple comparisons test or uncorrected Dunn’s test where appropriate. Cell count data were analysed with 2-tailed paired-samples t-tests with 95% confidence levels, using a Mann-Whitney test for non-parametric distribution. Gene expression data were analysed with 2-tailed paired-samples t-tests with 95% confidence levels.
All experiments were repeated at least three times with similar results obtained; sample sizes are shown in the respective figure legends.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study (images, quantitation data in the form of graphs and ATACseq data) are included in this published article and its supplementary information files. The source data are available in Supplementary Data 1 and the list of the called ATACseq peaks is available in Supplementary Data 2. The ATACseq data was deposited in GEO (Accession number GSE143763).
References
Ciau-Uitz, A., Monteiro, R., Kirmizitas, A. & Patient, R. Developmental hematopoiesis: ontogeny, genetic programming and conservation. Exp. Hematol. 42, 669–683 (2014).
Bonkhofer, F. et al. Blood stem cell-forming haemogenic endothelium in zebrafish derives from arterial endothelium. Nat. Commun. 10, 3577 (2019).
Gritz, E. & Hirschi, K. K. Specification and function of hemogenic endothelium during embryogenesis. Cell Mol. Life Sci. 73, 1547–1567 (2016).
Kalev-Zylinska, M. L. et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development 129, 2015–2030 (2002).
Swiers, G. et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat. Commun. 4, 2924 (2013).
Kissa, K. & Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 (2010).
Bertrand, J. Y. et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 (2010).
Boisset, J. C. et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464, 116–120 (2010).
Kissa, K. et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood 111, 1147–1156 (2008).
Davidson, A. J. & Zon, L. I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23, 7233–7246 (2004).
Murayama, E. et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975 (2006).
Hsu, A. P. et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood 121, 3830–3837 (2013). S3831-3837.
Wlodarski, M. W., Collin, M. & Horwitz, M. S. GATA2 deficiency and related myeloid neoplasms. Semin. Hematol. 54, 81–86 (2017).
Wlodarski, M. W. et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 127, 1387–1397 (2016). quiz 1518.
Tsai, F. Y. et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–226 (1994).
de Pater, E. et al. Gata2 is required for HSC generation and survival. J. Exp. Med. 210, 2843–2850 (2013).
Ling, K. W. et al. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200, 871–882 (2004).
Khandekar, M. et al. A Gata2 intronic enhancer confers its pan-endothelia-specific regulation. Development 134, 1703–1712 (2007).
Gao, X. et al. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 210, 2833–2842 (2013).
Gillis, W. Q., St John, J., Bowerman, B. & Schneider, S. Q. Whole genome duplications and expansion of the vertebrate GATA transcription factor gene family. BMC Evol. Biol. 9, 207 (2009).
Butko, E. et al. Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development 142, 1050–1061 (2015).
Liu, J., Jiang, J., Wang, Z., He, Y. & Zhang, Q. Origin and evolution of GATA2a and GATA2b in teleosts: insights from tongue sole, Cynoglossus semilaevis. PeerJ 4, e1790 (2016).
Yang, L., Rastegar, S. & Strahle, U. Regulatory interactions specifying Kolmer-Agduhr interneurons. Development 137, 2713–2722 (2010).
Zhu, C. et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development 138, 4555–4564 (2011).
Patterson, L. J. et al. The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109, 2389–2398 (2007).
Johnson, K. D. et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J. Clin. Invest. 122, 3692–3704 (2012).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Jin, S. W., Beis, D., Mitchell, T., Chen, J. N. & Stainier, D. Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005).
Meadows, S. M., Myers, C. T. & Krieg, P. A. Regulation of endothelial cell development by ETS transcription factors. Semin Cell Dev. Biol. 22, 976–984 (2011).
Wozniak, R. J. et al. Molecular hallmarks of endogenous chromatin complexes containing master regulators of hematopoiesis. Mol. Cell Biol. 28, 6681–6694 (2008).
Kawakami, K. et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 (2004).
Bassett, A. R., Tibbit, C., Ponting, C. P. & Liu, J. L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4, 220–228 (2013).
Lawson, N. D. et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675–3683 (2001).
Dobrzycki, T., Krecsmarik, M., Bonkhofer, F., Patient, R. & Monteiro, R. An optimised pipeline for parallel image-based quantification of gene expression and genotyping after in situ hybridisation. Biol. Open 7, bio031096 (2018).
Wilkinson, R. N. et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell 16, 909–916 (2009).
Bertrand, J. Y. et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147–4156 (2007).
Willett, C. E., Zapata, A. G., Hopkins, N. & Steiner, L. A. Expression of zebrafish rag genes during early development identifies the thymus. Dev. Biol. 182, 331–341 (1997).
Lin, H.-F. F. et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood 106, 3803–3810 (2005).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Liu, Z. et al. Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nat. Commun. 10, 1839 (2019).
Traver, D. et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 4, 1238–1246 (2003).
Athanasiadis, E. I. et al. Single-cell RNA-sequencing uncovers transcriptional states and fate decisions in haematopoiesis. Nat. Commun. 8, 2045 (2017).
Macaulay, I. C. et al. Single-cell RNA-sequencing reveals a continuous spectrum of differentiation in Hematopoietic Cells. Cell Rep. 14, 966–977 (2016).
Sanalkumar, R. et al. Mechanism governing a stem cell-generating cis-regulatory element. Proc. Natl Acad. Sci. USA 111, E1091–1100 (2014).
Rodrigues, N. P. et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 106, 477–484 (2005).
Ma, D., Zhang, J., Lin, H.-f, Italiano, J. & Handin, R. I. The identification and characterization of zebrafish hematopoietic stem cells. Blood 118, 289–297 (2011).
Westerfield, M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio). 5th edn, (Univ of Oregon Press, 2007).
Chi, N. C. et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 22, 734–739 (2008).
Howe, K. et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498–503 (2013).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Birnbaum, R. Y. et al. Coding exons function as tissue-specific enhancers of nearby genes. Genome Res. 22, 1059–1068 (2012).
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Gagnon, J. A. et al. Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS ONE 9, e98186 (2014).
Xu, H. et al. Genome-wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT-qPCR. J. Fish. Biol. 88, 2095–2110 (2016).
Hu, Y., Xie, S. & Yao, J. Identification of novel reference genes suitable for qRT-PCR normalization with respect to the Zebrafish developmental stage. PLoS ONE 11, e0149277 (2016).
Stachura, D. L. & Traver, D. Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 133, 11–53 (2016).
Jowett, T. & Yan, Y. L. Double fluorescent in situ hybridization to zebrafish embryos. Trends Genet 12, 387–389 (1996).
Thompson, M. A. et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 197, 248–269 (1998).
Monteiro, R. et al. Transforming growth factor beta drives hemogenic endothelium programming and the transition to hematopoietic stem cells. Dev. Cell 38, 358–370 (2016).
Acknowledgements
We thank the staff of the Biomedical Services Units (Oxford, Birmingham and Rotterdam) for fish husbandry. We thank Kevin Clark and Sally-Ann Clark from the WIMM flow cytometry facility for cell sorting. The flow cytometry facility is supported by the MRC HIU, MRC MHU (MC_UU_12009), NIHR Oxford BRC and John Fell Fund (131/030 and 101/517), the EPA fund (CF182 and CF170). We thank the Wolfson Imaging Centre Oxford for imaging. The Centre is supported by a Wolfson Foundation (grant 18272), and an MRC/BBSRC/EPSRC grant (MR/K015777X/1) to MICA – Nanoscopy Oxford. Both facilities were supported by the WIMM Strategic Alliance awards G0902418 and MC_UU_12025. We thank Fatma Kok and Douglas Vernimmen for critical reading of the manuscript. We thank Feng Liu for the generous gift of the fli1a-NICD:GFP construct. This research was supported by the British Heart Foundation (BHF Oxford CoRE and BHF IBSR Fellowship FS/13/50/30436 to R.M. and M.K.), by a Wellcome Trust Chromosome and Developmental Biology PhD Scholarship (#WT102345/Z/13/Z. to T.D.) and by the MRC MHU programme (MC_UU_12009/8 to R.P.).
Author information
Authors and Affiliations
Contributions
T.D., M.K., C.K., E.P. and R.M. designed the study. T.D., M.K., C.K., J.P-Z., K.G., C.B.M. and R.M. performed experiments and analyzed the data. J.P-Z., B.F. and K.G. performed experiments. R.R. performed the bioinformatics analyses, T.D. and R.M. wrote the paper and R.P., E.P. and R.M. edited the paper. R.P., E.P. and R.M. secured funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dobrzycki, T., Mahony, C.B., Krecsmarik, M. et al. Deletion of a conserved Gata2 enhancer impairs haemogenic endothelium programming and adult Zebrafish haematopoiesis. Commun Biol 3, 71 (2020). https://doi.org/10.1038/s42003-020-0798-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-0798-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.