Abstract
The remains of ovarian follicles reported in nine specimens of basal birds represents one of the most remarkable examples of soft-tissue preservation in the Early Cretaceous Jehol Biota. This discovery was immediately contested and the structures alternatively interpreted as ingested seeds. Fragments of the purported follicles preserved in an enantiornithine (STM10-12) were extracted and subjected to multiple high-resolution analyses. The structures in STM10-12 possess the histological and histochemical characteristics of smooth muscles fibers intertwined together with collagen fibers, resembling the contractile structure in the perifollicular membrane (PFM) of living birds. Fossilized blood vessels, very abundant in extant PFMs, are also preserved. Energy Dispersive Spectroscopy shows the preserved tissues primarily underwent alumino-silicification, with minor mineralization via iron oxides. No evidence of plant tissue was found. These results confirm the original interpretation as follicles within the left ovary, supporting the interpretation that the right ovary was functionally lost early in avian evolution.
Similar content being viewed by others
Introduction
Since the discovery of the first feathered birds and non-avian dinosaurs from the Jehol Biota of northeastern China in the 1990s, thousands of specimens have been reported1. These volcanolacustrine deposits2 represent a taphonomic window through which exceptionally well-preserved specimens have revealed important aspects of the biology of a diversity of organisms also including salamanders, pterosaurs, fish, and mammals1,3,4. Specimens are typically more than 90% complete, fully articulated and often preserving soft tissues1. In addition to feathers, soft tissue remnants reported from Jehol birds include skin5, cartilage and ligaments6, lungs7, and perhaps most controversial of all, ovarian follicles8,9.
These follicles were described in nine specimens of early birds, including one specimen of the basal, long bony-tailed Jeholornis, one specimen of the confuciusornithiform Eoconfuciusornis, and seven specimens of enantiornithines, the dominant clade of land birds during the Cretaceous5,8,9,10. More precisely, the follicles were interpreted as remnants of the perifollicular membrane (PFM) of pre-ovulatory ovarian follicles, a connective tissue that surrounds the yolk-filled oocytes prior to ovulation in extant birds9. In all specimens, the purported follicles are preserved as agglomerated, circular, or oval structures on the left side of the body cavity, which led to the conclusion that the earliest birds were like their extant relatives in having only one functional ovary (the left)8,9. Together with information from an oviraptorosaur with two eggs preserved dorsal to its pubic symphysis11, this suggested that the loss of function of the right ovary occurred very close to the dinosaur–bird transition9 supporting existing hypotheses that this loss was linked to the evolution of flight (i.e., being supposedly aerodynamically advantageous, in that the loss of one ovary would have reduced mass and facilitated flight during reproductive periods12). Additionally, because all purported follicles are approximately the same (relative) size in all specimens, it was hypothesized that basal birds had a low degree of follicular hierarchy due to slow yolk deposition reflecting their relatively lower basal metabolic rate8, which is unlike the condition in modern birds in which the follicular hierarchy is pronounced. This inference was supported by the discovery of the follicles preserved in Eoconfuciusornis, which showed a stronger hierarchy than that in Jeholornis and enantiornithines, consistent with interpretations that confuciusornithiforms had relatively elevated metabolic rates when compared to other basal avian lineages5.
The identification of fossilized ovarian follicles in Jehol birds has been controversial13,14,15,16,17. Because follicles are soft tissue structures (i.e., originally unbiomineralized) mostly consisting of viscous yolk surrounded by a thin PFM, it was argued that they are unlikely to fossilize and an alternative hypothesis was proposed, interpreting these structures as ingested seeds (i.e., stomach contents, or cololites)13,14,15. The original identification of the follicles and the secondarily proposed alternative hypothesis were both based entirely on macroscopic morphological observations and preserved anatomical location.
These two conflicting hypotheses can easily be tested at the tissue level. If the purported follicles are indeed remnants of a PFM, they should present the same histological characteristics as the homologous tissues forming the PFM in the ovaries of extant, reproductively active birds. The PFM (or theca) surrounds the yolk-filled oocytes and links them to the ovarian medulla18. The three main components of a PFM are collagen fibers, smooth muscle fibers, and blood vessels18,19,20,21. On the other hand, the tissues forming seeds (or any type of plant material) are completely different and their cells possess a distinct cell wall absent in all animal cells22.
To investigate these competing hypotheses, we conducted an in-depth microscopic analysis of fragments extracted from the purported follicles preserved in an enantiornithine from the Shandong Tianyu Museum of Nature, STM10–12, originally described by O’Connor et al.8. We used standard ground-sectioning methods, scanning-electron microscopy (SEM), and paraffin histology conducted on demineralized samples to compare the morphology and histochemistry of the fossilized structures with tissues composing pre-ovulatory follicles in an extant hen (Gallus gallus domesticus; the extant homologue for this study), and to published data on seed tissues22. Histological observations were paired with energy-dispersive spectroscopy (EDS) in order to further understand the chemical alterations that these fossilized soft-tissues underwent during diagenesis. Our results do not show any evidence of plant tissues, but instead reveal histological and histochemical data consistent with fossilized smooth muscles fibers, collagen fibers, and blood vessels. Together, these fossilized tissues show the same organization and characteristics of the contractile structure that expels the oocyte during ovulation in living birds19,20. This study confirms both the original interpretation as follicles within the left ovary, and that the right ovary was functionally lost early in avian evolution8,9.
Results
Surface texture
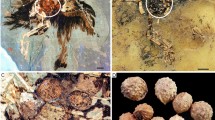
The ovarian follicles reported in the nine basal bird specimens all differ in gross morphology and surface texture (summarized in Supplementary Table 1). In STM10–12 (Fig. 1a) at least five circular structures are visible in the slab (Fig. 1b), and at least three are visible in the counterslab (Fig. 1c). These traces consist of pale pink impressions within a lighter sediment. They are organized in clusters similarly to follicles in extant birds, but unlike the modern avian condition (Supplementary Fig. 1a) they all have approximately the same size (average diameter of 7.7 mm; Fig. 1b, c). Some areas show a cracked surface, with a brown or dark orange tint (Fig. 1d). Two fragments were extracted from the counterslab and used for tissue analysis (Fig. 1d). Prior to being embedded and sectioned, the surface of the first fragment (Fragment 1; Fig. 1d) was observed under the SEM, which revealed it is coated in a thick layer of glue/consolidant, which was applied during fossil preparation prior to this study (Supplementary Fig. 2).
General view of the slab a and close-up on the purported ovarian follicles, all approximately the same size (pink arrows in b). Close-ups on these same structures (pink arrows) in the counterslab c. Two fragments were used for different analytical methods d. The first fragment (orange outline) was prepared using ground sectioning methods. The second fragment (blue outline) was demineralized and processed for paraffin histology. Abbreviations: Frag. fragment, sed sediment.
Histology and histochemistry
General overview: A ground section of the first fragment (Fragment 1; Fig. 1d) shows three main characteristics: (1) the above-mentioned top layer of consolidant (blue arrow); (2) a light brown material below it (pink arrow); and (3) a bottom layer of sediment (Fig. 2a). The consolidant is transparent and between 20 and 50 µm thick. It is covered by crystals in a few areas (Fig. 2a), and faintly birefringent under polarized light (Fig. 2b). Right below it, the brown material does not possess any clearly identifiable tissue structures nor cells, even when observed at high magnification under transmitted light (Fig. 2c). Under polarized light, this brown material is strongly birefringent (Fig. 2b). However, SEM revealed that this material is composed of fibers (compare Fig. 1c, d). Right below this fibrous material the top layer of the sediment contains a black tint resembling decayed organic matter (Fig. 2a, b).
Ground-section images a–c, SEM images d–f and demineralized paraffin slides of STM10–12 g–j reveal structures consistent with extant collagen fibers and smooth muscle fibers k, l. a A cross section of the first fragment seen under transmitted light shows a transparent layer of consolidant (blue arrow), and a brown material underneath it representing the preserved soft-tissues (pink arrow). b Same image under the polarized light. c Close-up on the consolidant and the brown material. d Complementary SEM image, showing fibers in the brown material (pink arrows). e Close-up showing undulating, thick fibers, consistent in size with smooth muscle fibers. f Another close-up showing much thinner fibers between 0.3 and 1 µm thick, consistent with the size of collagen fibers. g Unstained paraffin slide of the second fragment of STM10–12, showing a fibrous material (pink arrow), covered by glue (blue arrow). h Close-up of red square in g. i Close-up of red square in h. j Adjacent slide of STM10–12 stained with Masson’s trichrome. k Extant PFM tissues from an ovarian follicle near ovulation (extant hen) stained with Masson’s trichrome. It shows smooth muscle fibers (SMFs, pink arrows) and collagen fibers (CFs; green arrows) organized into a chordae, and some blood vessels. l Close-up on the lower red square in k. m Close-up on the upper red square in k showing some CFs with their typical crimp-waveform arrangement. The fossil material shows the same morphology and staining pattern as extant SMFs and CFs organized into a chordae. Images e, i, j, l, m are shown at the same scale for direct comparison. Additional abbreviations: BV blood vessels; OM organic matter, RBCs red blood cells, sed sediment.
Fossilized fibrous tissues: When the fibrous material is observed at high resolution under the SEM, densely packed, undulating fibers of two different size ranges can be seen (Fig. 2e, f): larger fibers with a thickness between 2 and 3 µm (Fig. 2e), and smaller fibers between 0.3 and 1 µm thick (Fig. 2f).
The second sample (Fragment 2; Fig. 1d) was demineralized and paraffin sections were made at 5 µm (Fig. 2g–j). The demineralized sample revealed densely packed, undulating fibrous material (pink arrow; Fig. 2g–i).
Morphological and histochemical comparison with extant fibrous tissues: We compared the fossilized undulating fibers seen in STM10–12 to the fibers composing the PFM of an ovarian follicle near ovulation from an extant hen (Fig. 2k–m). The extant PFM was stained with a modified Masson’s trichrome (a common connective tissue stain23), revealing blood vessels surrounded by a connective tissue made of collagen fibers (stained green) and larger smooth muscle fibers (stained pink) (Fig. 2k–m). This fibrous structure, called the chordae, contracts to expel the yolk-filled oocyte during ovulation19,20,21 (Fig. 2k). Collagen fibers are easily recognizable by their small size and their wavy organization, referred to as a crimp waveform arrangement (e.g., ref. 24; Fig. 2l, m).
At low magnification, the overall shape and undulating pattern of the fossilized fibrous material is consistent in size and morphology to the chordae in our extant Gallus sample (compare Fig. 2h with Fig. 2k). The two different size ranges of fibers seen in the fossil under SEM (Fig. 2e, f) are also consistent with the size of the smooth muscle fibers and collagen fibers in the extant material (Fig. 2l, m). When stained with Masson’s trichrome (Fig. 2j), the fossilized fibers also showed a staining pattern very similar to that seen in the extant chordae (compare Fig. 2j, l, m, shown at the same scale). Most of the fossilized fibrous material stained pink, and a few smaller fibers stained green and showed the typical crimp waveform arrangement unique to collagen fibers (Fig. 2j).
Fossilized vessel-like structures and comparison with extant vessels: Additional structures were observed in the paraffin slides made from the demineralized sample of STM10–12, which were not seen in the ground-section nor the SEM images. Near the fossilized chordae some hollow, tubular structures can be seen (Fig. 3a–e). These structures sometimes show a branching pattern (Fig. 3c) and have walls (Fig. 3d, e). These structures present the same morphological characteristics of extant, avian blood vessels (Fig. 3f). The size of these vessel-like structures falls within the size range of extant blood vessels observed in an extant PFM (e.g., compare Fig. 3d with Fig. 3f, shown at the same scale).
a Unstained slide of STM10–12 showing the preserved fibrous soft-tissues (pink arrow). b Close-up showing a structure resembling a blood vessel (BV) near the fossilized chordae. c Close-up in another slide showing bifurcation (black arrows) in a BV-like structure. d Close-up from another unstained slide showing BV-like structures with walls and internal material. e Corresponding image under the polarized light. f Unstained PFM from an extant hen showing blood vessels, red blood cells (red arrows), their sub-endothelial connective tissue (SECT) and intravascular material (IVM). Images d–f are shown at the same scale for direct comparison.
The walls of the fossilized blood vessels are birefringent under polarized light (Fig. 3d) and are morphologically similar to the sub-endothelial connective tissue observed in extant blood vessels (Fig. 3f). The inside of the tubular vessels preserves an amorphous substance that resembles intravascular material (IVM) sometimes visible in histological sections of extant blood vessels (compare the IVM in Fig. 3d with Fig. 3f), most likely representing fossilized blood breakdown products and/or plasma (e.g., see Fig. 1h in Schweitzer et al.25).
Energy-dispersive spectroscopy: The soft tissues observed in the ground-section (i.e., the fossilized chordae with blood vessels) were chemically analyzed. EDS shows the preserved soft-tissues are enriched in silicon, oxygen, aluminum, with low amounts of carbon and iron. Compared to the sediment there is a net enrichment in aluminum and iron in the soft-tissues (Fig. 4). These results suggest that during diagenesis the soft-tissues primarily underwent alumino-silicification, with slight mineralization via iron oxides (Fig. 4). The chemical signal of the consolidant is very different from the soft-tissues and consists mostly of carbon and oxygen (Fig. 4).
a SEM image (same image as Fig. 2d) with yellow line showing where the EDS profile was taken. b Enlargement and horizontalization of a. The EDS profile was followed along the external layer of glue, the soft tissue layer with preserved fibers, and the sediments below them. c EDS profile showing the chemistry of all features (glue, soft tissues, and sediments) of major elements in counts per second (cps). The layer of glue is highly enriched in carbon (C), followed by low amounts of oxygen (O), silicon (Si) and aluminum (Al). The Si and Al signals seen in the glue are most likely a contaminant from the sediment and/or soft tissues beneath. The Si signal may also come from the glass of the ground-section. The soft tissues (concluded to be part of the original perifollicular membrane) beneath the consolidant show a net drop in C content, but a clear increase in Si, O, and Al. The source of Al in the soft-tissues may come from clay minerals within the sediments below, and it is possible that direct precipitation of authigenic clays onto the tissues occurred shortly after death. The soft-tissues are also enriched in Iron (Fe, blue line) when compared to the sediments. There are also negligible amounts of magnesium (Mg), potassium (K), calcium (Ca), and titanium (Ti) in the sample.
Discussion
When first described, it was hypothesized that the circular traces preserved in basal birds from the Jehol Biota represented remnants of the PFM of mature or nearly mature follicles within the left ovary9. The absence of calcified eggshell in the oviduct suggested that ovulation had not begun in any specimen. In one enantiornithine (STM29-8), the unusual surface texture in the purported follicles was interpreted as the imprints of a well-developed network of blood vessels within a highly vascularized PFM9 (Supplementary Fig. 3).
The results of our analyses support identification of the remains preserved in enantiornithine STM10–12 as remnants of the ovary and a vascularized PFM. The tissues in STM10–12 present the same morphological and histochemical characteristics as those of an avian chordae, a contractile structure made of intertwined collagen fibers with smooth muscle fibers that expel the oocyte during ovulation in extant birds (Fig. 2). STM10–12 also preserves structures morphologically consistent with extant blood vessels (Fig. 3). Given that the analyzed fragments of purported follicles in STM10–12 present virtually all the tissue characteristics (i.e., appropriate size, morphology, and histochemistry) of the three main components found in extant PFMs (i.e., smooth muscle fibers, collagen fibers, and blood vessels) the most parsimonious and plausible conclusion is that the circular structures in STM10–12 are indeed the fossilized remnants of pre-ovulatory ovarian follicles, also consistent with their preserved anatomical location. Fossilized structures morphologically consistent with collagen fibers and muscle fibers have been previously identified in numerous other Mesozoic specimens (e.g., refs. 26,27,28,29,30,31). This study contributes to the mounting evidence that such tissue components can preserve in deep-time.
The fossilized follicles in STM10–12 are by no means completely preserved, but rather represent fragments of these structures. We found no histological evidence of other tissues that are found in the PFM of extant pre-ovulatory follicles, such as non-collagenous fibers of the inner perivitelline membrane, granulosa cells, nerve fibers32, and the ovarian surface epithelium (Supplementary Fig. 1).
Fossilized blood vessels have been previously reported in some specimens of Mesozoic dinosaurs and Cenozoic turtles (e.g., refs. 33,34,35,36). The vessels in STM10–12 are not consistent in morphology with fungal hyphae in that they lack septae and fungal hyphae are much smaller (see refs. 33,34). Given their size and morphology, the most logical interpretation is that these structures are remnants of original blood vessels belonging to an originally highly vascularized PFM.
The fossilization of blood vessels is apparently more common than generally recognized in material from the Mesozoic and these structures have already been thoroughly documented both morphologically and chemically (e.g., refs. 26,29,33,34,37,38,39). In previous studies, fossil blood vessels were mostly observed in three dimensions (3D), photographed as ‘floating’ material in demineralizing solutions, whereas in this study the blood vessels were observed in 2D sections exposing the blood vessels through longitudinal cuts (Fig. 3). In blood vessels analyzed in 3D, branching is also commonly observed (e.g., see refs. 33,38,40) but in contrast, in sectioned vessels branching is much less common (e.g., see Fig. 3f–h), which may explain why very few branching patterns were observed in our sample (only in Fig. 3c).
Noteworthy, blood vessels in STM10–12 were only visible in the demineralized paraffin slides (Fig. 3) but not in the ground-sections nor the SEM images (Fig. 2). A possible logical explanation is that this is due to differences in tissue compaction and distortion between the samples in the ground-sections and the paraffin slides. In the ground sections, the tissues were embedded in resin without being demineralized. Resin-embedded tissues are tightly compacted (Fig. 2d) and do not undergo any significant distortion during preparation. On the other hand, demineralized tissues that get embedded in paraffin go through multiple distortion and tearing events (i.e., during demineralization, processing through different solutions, but especially after being cut on a microtome and placed on top of warm water in the water bath prior to mounting on glass slides, see Supplementary Methods). These distortions create an artificial ‘decompaction’ of the fossil tissues in the paraffin slides (which occurs commonly even while making slides of extant tissues), and this is most likely what enabled the visualization of blood vessels in STM10–12 (Figs. 2g–j and 3) that were not directly visible in ground-sections nor through SEM (Fig. 2d). These results suggest that the three methods employed here (ground-sectioning, SEM, and paraffin histology) yield complementary information and, when used together, can help to provide more rigorous identifications and clarify our understanding of fossilized soft-tissues.
EDS showed that the fossilized soft-tissues preserved in STM10–12 underwent alumino-silicification (Fig. 4). This same process has been reported in fossilized branchiopod (clam shrimp) eggs also from the Jehol Biota, where all the envelopes were made of calcium phosphate but some of the eggs had their internal contents replaced by alumino-silicates41. An explanation for the mechanism of alumino-silicate replacement was not provided for the Jehol clam shrimp eggs41, but we suggest that clay minerals from sediments were involved. Clay minerals have been determined to be important agents in the fossilization of soft-tissues in other settings (such as the Ordovician Soom Shale of South Africa and the Cambrian Burgess Shale of Canada)42,43. In these cases, the soft-tissues were replaced rapidly after death by authigenic clay minerals (via direct precipitation of authigenic clays onto the tissues). It is the most logical explanation for the process of alumino-silicification seen in the tissues of STM10–12, and this process may have happened rapidly after death before extensive tissue decay.
EDS also revealed an enrichment in iron, indicating that the soft-tissues may also have experienced some limited mineralization with iron oxides (Fig. 4, see the low iron levels). The mineralization of soft-tissues via iron oxides (such as goethite and biogenic iron oxyhydroxide) has been reported in Mesozoic dinosaurs38 and a similar process may have occurred as well in STM10–12. A potential source for this iron may be the pyroclastic flows that intermittently interrupted the deposition of lacustrine sediments, and/or microbial mats, as proposed for Jehol invertebrates44,45. Moreover, based on our new data, we also suggest that another source of this iron may be the hemoglobin (a protein found in red blood cells) coming directly from the blood vessels of the organism itself. An experimental study on ostrich blood vessels demonstrated that iron and oxygen from hemoglobin play a key role in tissue stability and in the exceptional preservation of soft-tissues in deep-time38. This hypothesis could be tested in the future with immunohistochemistry and antibodies raised against avian hemoglobin.
Although pyritization (via iron sulfides) has been demonstrated in the soft-body parts of Jehol insects and hypothesized to play an important role in the preservation of soft-tissues in Jehol fossils44,45, the EDS data here do not show any sulfur in the sample, meaning STM10–12 underwent a different fossilization process and that pyritization is simply one of the many processes involved in soft-tissue preservation in the Jehol paleolakes.
All of the histological and histochemical data collected here (Figs. 1–4) demonstrate the exceptional preservation of the soft-tissues in STM10–12 (i.e., fossilized chordae and blood vessels from the PFM), but more precise and more specific chemical analyses (such as synchrotron-FTIR, or immunohistochemistry) are needed to fully characterize the preservation of these tissues at a deeper molecular level.
Until this study, interpretations of the purported follicles as ingested seeds (e.g., see Supplementary Fig. 4) indeed represented a viable alternative hypothesis13,14. Testing these competing hypotheses was imperative to our understanding of both the evolution of the paravian reproductive system (i.e., to confirm whether or not early birds indeed had only one functional ovary like extant birds and lacked strong follicular hierarchy) and digestive system (as yet there is no direct evidence regarding the diet of enantiornithines in the Jehol46,47).
Many morphological arguments have previously been raised against the interpretation of these remains as ingested seeds5. For example, the preserved structures also lack the surficial ornamentation observed in most fossilized seeds (Supplementary Fig. 4). Furthermore, the tissues here identified in STM10–12 (smooth muscle fibers, collagen fibers, and blood vessels; Figs. 2 and 3) are strictly animal tissues and are non-existent in plants. None of the histological slides of STM10–12 reveal tissues reminiscent of fossilized plant material with their characteristic cell walls22,48, from either gymnosperms or angiosperm seed or fruit tissues (e.g., cuticle, seed coat or testa, internal integument layers, and embryonic tissues; e.g., refs. 22,49,50). Although our original intent was to directly compare the tissues in STM10–12 with a fossil seed preserved in the stomach of the holotype of Jeholornis prima (Supplementary Fig. 4), close examination showed that the seeds in this specimen are only impressions, and thus cannot be used for proper comparison with seed tissues. Lastly, the STM10–12 samples were also checked for the presence of phytoliths, which are microscopic structures made of silica that are found in plant tissues and can persist for millions of years after the decay of the plant51, but none were found.
Although the preservation of ovarian follicles in STM10–12 is confirmed, this hypothesis needs to be independently tested in each individual specimen (Supplementary Table 1); as yet, it is still possible some of the purported follicles in other specimens may represent ingested seeds. Confirmation of follicle preservation in one specimen does nonetheless put an end to the controversy regarding whether or not such remains can preserve and confirms that only a single functional ovary was present in at least some non-neornithine avians8,9. Additionally, our results support paleobiological hypotheses based upon the original identification, such as observations regarding the relative sizes of these ovarian follicles. Compared to modern birds only subtle size variations are observed in the fossilized follicles of Jehol birds (meaning follicular hierarchy was absent), leading to the inference that in basal birds yolk deposition occurred much more slowly than in extant bird due to the lower metabolic rates of non-ornithuromorph birds8,9. This study reveals that this was true at least for enantiornithine STM10–12, which suggests this may be similarly true about other non-ornithuromorph birds in which slow growth rates are observed through osteohistology. It is likely that a strong follicular hierarchy is a derived feature of a subset of the Ornithuromorpha (because some of them still retain plesiomorphically slower growth rates), but only direct evidence with preserved follicles in Cretaceous ornithuromorphs could confirm this hypothesis.
The Early Cretaceous Jehol Biota of China preserves one of most extraordinary extinct fauna and flora ever discovered, revealed through a taphonomic environment that was extremely conducive to the fossilization of both hard and soft-tissues1,4. Few histological analyses on preserved soft-tissues exist due to the destructive nature of most of these methods. However, our results demonstrate that these types of analyses can eliminate doubt and help to further understand preservation. Previous taphonomic studies conducted on Jehol invertebrates showed that alumino-silicification and pyritization help preserve soft-tissues in the Jehol41,45. In STM10–12, no pyritization was found, but the tissues apparently underwent alumino-silicification and a slight mineralization with iron, potentially iron oxides. This suggests that soft-tissue fossilization in the Jehol is case-specific and that varied mechanisms were involved. In the case of animal tissues, it is possible that preservation of soft tissues was facilitated by endogenous iron and oxygen from the hemoglobin in blood26,38. The abundant blood vessels in the PFM may explain the high preservation potential of ovarian follicles, although this hypothesis cannot explain why other tissues and organs that are also highly vascularized are not preserved in the same specimens.
In STM10–12 and all other specimens with purported follicles, the overall spherical shape of the follicles is preserved in 2D. Therefore, it is also possible that organic remnants from the original spherical yolk and/or the inner perivitelline membrane are preserved, but extensive further analyses (using different methods such as immunohistochemistry or spectroscopy) are required to confirm this. Additionally, the most external tissue covering preovulatory follicles in extant birds is made of pancytokeratin18, a hydrophobic protein with a high potential for fossilization52. We propose that this hydrophobic molecule may have acted as a barrier and also facilitated the exceptional preservation of follicles. Much more research is necessary to confirm this hypothesis, and more studies on preserved soft-tissues in the Jehol are necessary to further shed light on the modes of tissue preservation through deep-time.
Methods
Ground-sectioning
The two fragments from the counterslab of STM10–12 were extracted from the fossil with a scalpel. One sample was observed under the SEM (Supplementary Fig. 2) and then embedded in EXAKT Technovit 7200 (Norderstedt, Germany) one-component resin, and cured for 24 h, cut using an EXAKT 300CP accurate circular saw, and then ground and polished using the EXAKT 400CS grinding system (Norderstedt, Germany) until the desired optical contrast was reached (about 50 μm). Sections were observed under transmitted and polarized light using a Nikon eclipse LV100NPOL, and photographed with a DS-Fi3 camera and the software NIS-Element v4.60. Paraffin sections (see below) were observed and photographed with this same system.
SEM-EDS
The ground-section was analyzed using SEM at the Chinese Academy of Geological Sciences with a FEI Quanta 450 (FEG) at 20 kV (Fig. 2). Both BSE and SE modes (backscattered electrons and secondary electrons) were applied. The EDS profile were measured as a line, continuous between different layers (Fig. 4).
Paraffin histology
A few extant samples of whole ovarian follicles (‘large white follicles’) and the PFM of a more mature follicle (‘hierarchical follicle’) completely filled with yolk were dissected from a gravid female chicken obtained commercially (Supplementary Fig. 1). These extant tissues were fixed in 10% neutral buffered formalin (NBF) for 48 h. Demineralization of the extant tissues was not necessary as they are all unbiomineralized soft tissues. The second fragment from STM10–12 was embedded in 3% agar (Becton Dickinson Cat# 214530) (for stabilization) and demineralized (without NBF) in 500 mM EDTA; pH 8.0 for 3 weeks with a solution changes every 2–3 days. Demineralization of the fossil tissues was made simply to comply with the microtome blades and paraffin protocols. All tissues (extant and fossils) were subjected to routine dehydration, to clearing in xylene, and to paraffin infiltration and embedding (Supplementary Methods). Sections were cut at 5 µm on a rotary microtome (Leica Biosystems RM2265), placed into a warm water bath (at about 44 °C) and mounted on charged slides (Superfrost Plus, Fisher Scientific). Most slides of STM10–12 were left unstained: they were simply deparaffinized in different solutions of xylene for about 15 min and cover-slipped with mounting medium (Permount, Fisher Scientific). Some slides (of STM 10–12 and extant tissues) were stained with a modified Masson’s trichrome23 (Supplementary Methods). Contamination between fossil and extant tissues has been avoided (see Supplementary Methods).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
STM10–12 is reposited at the Shandong Tianyu Museum of Nature in Linyi City. Ground-sections and paraffin sections of STM10–12 fragments are currently reposited at the Institute of Vertebrate Paleontology and Paleoanthropology in Beijing. All data are available upon reasonable request.
References
Zhou, Z. The Jehol Biota, an Early Cretaceous terrestrial Lagerstätte: new discoveries and implications. Natl Sci. Rev. 1, 543–559 (2014).
Jiang, B., Harlow, G. E., Wohletz, K., Zhou, Z. & Meng, J. New evidence suggests pyroclastic flows are responsible for the remarkable preservation of the Jehol biota. Nat. Commun. 5, 3151 (2014).
Zhou, Z. Evolutionary radiation of the Jehol Biota: chronological and ecological perspectives. Geol. J. 41, 377–393 (2006).
Zhou, Z., Barrett, P. M. & Hilton, J. An exceptionally preserved Lower Cretaceous ecosystem. Nature 421, 807 (2003).
Zheng, X. et al. Exceptional preservation of soft tissue in a new specimen of Eoconfuciusornis and its biological implications. Natl Sci. Rev. 4, 441–452 (2017).
Jiang, B. et al. Cellular preservation of musculoskeletal specializations in the Cretaceous bird Confuciusornis. Nat. Commun. 8, 14779 (2017).
Wang, X. et al. Archaeorhynchus preserving significant soft tissue including probable fossilized lungs. Proc. Natl Acad. Sci. USA 115, 11555–11560 (2018).
O’Connor, J. K., Zheng, X., Wang, X., Wang, Y. & Zhou, Z. Ovarian follicles shed new light on dinosaur reproduction during the transition towards birds. Natl Sci. Rev. 1, 15–17 (2013).
Zheng, X. et al. Preservation of ovarian follicles reveals early evolution of avian reproductive behaviour. Nature 495, 507 (2013).
Wang, Y. et al. A new Jehol enantiornithine bird with three-dimensional preservation and ovarian follicles. J. Vertebrate Paleontol. 36, e1054496 (2016).
Sato, T., Cheng, Y.-n, Wu, X.-c, Zelenitsky, D. K. & Hsiao, Y.-f A pair of shelled eggs inside a female dinosaur. Science 308, 375–375 (2005).
Taylor, T. How an eggshell is made. Sci. Am. 222, 88–97 (1970).
Chiappe, L. M. & Qingjin, M. Birds of Stone: Chinese Avian Fossils from the Age of Dinosaurs (JHU Press, 2016).
Mayr, G. & Manegold, A. Can ovarian follicles fossilize? Nature 499, E1 (2013).
Mayr, G. Avian Evolution: the Fossil Record of Birds and its Paleobiological Significance (John Wiley & Sons, 2016).
Deeming, D. The fossil record and evolution of avian egg nesting and incubation. In Nests, Eggs, and Incubation (ed. Deeming, D. C.) 8–15 (Oxford University Press, Oxford, 2015).
Varricchio, D. J. & Jackson, F. D. Reproduction in Mesozoic birds and evolution of the modern avian reproductive mode. Auk 133, 654–684 (2016).
Apperson, K., Bird, K., Cherian, G. & Löhr, C. Histology of the ovary of the Laying Hen (Gallus domesticus). Vet. Sci. 4, 66 (2017).
Callebaut, M. The ovarian chordolacunar system in birds. Arch. Biol. 99, 1–15 (1988).
Phillips, R. E. & Warren, D. C. Observations concerning the mechanics of ovulation in the fowl. Journal of Experimental Zoology 76, 117–136 (1937).
Rodler, D., Stein, K. & Korbel, R. Observations on the right ovary of birds of prey: a histological and immunohistochemical study. Anat. Histol. Embryol. 44, 168–177 (2015).
Taylor, E. L., Taylor, T. N. & Krings, M. Paleobotany: The Biology and Evolution of Fossil Plants (Elsevier Science, 2009).
Witten, P. E. & Hall, B. K. Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. J. Anat. 203, 435–450 (2003).
Niven, H., Baer, E. & Hiltner, A. Organization of collagen fibers in rat tail tendon at the optical microscope level. Collagen Relat. Res. 2, 131–142 (1982).
Schweitzer, M. H. et al. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 316, 277–280 (2007).
Wiemann, J. et al. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 9, 4741 (2018).
Avci, R. et al. Preservation of bone collagen from the late Cretaceous period studied by immunological techniques and atomic force microscopy. Langmuir 21, 3584–3590 (2005).
Schweitzer, M. H. et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 324, 626–631 (2009).
Pawlicki, R., Korbel, A. & Kubiak, H. Cells, collagen fibrils and vessels in dinosaur bone. Nature 211, 655–657 (1966).
Chin, K. et al. Remarkable preservation of undigested muscle tissue within a Late Cretaceous tyrannosaurid coprolite from Alberta, Canada. Palaios 18, 286–294 (2003).
McNamara, M. et al. Organic preservation of fossil musculature with ultracellular detail. Proc. R. Soc. Lond. B 277, 423–427 (2010).
Gilbert, A. Innervation of the ovarian follicle of the domestic hen. Q. J. Exp. Physiol. Cogn. Med. Sci. 50, 437–445 (1965).
Schweitzer, M. H., Wittmeyer, J. L. & Horner, J. R. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proc. R. Soc. B 274, 183–197 (2007).
Schweitzer, M. H., Wittmeyer, J. L., Horner, J. R. & Toporski, J. K. Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science 307, 1952–1955 (2005).
Cadena, E. Microscopical and elemental FESEM and Phenom ProX-SEM-EDS analysis of osteocyte-and blood vessel-like microstructures obtained from fossil vertebrates of the Eocene Messel Pit, Germany. PeerJ 4, e1618 (2016).
Cadena, E. A. & Schweitzer, M. H. A Pelomedusoid turtle from the Paleocene–Eocene of Colombia exhibiting preservation of blood vessels and osteocytes. J. Herpetol. 48, 461–465 (2014).
Pawlicki, R. & Nowogrodzka-Zagórska, M. Blood vessels and red blood cells preserved in dinosaur bones. Ann. Anat.-Anatomischer Anz. 180, 73–77 (1998).
Schweitzer, M. H. et al. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. R. Soc. B 281, 20132741 (2014).
Cleland, T. P. et al. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. J. Proteome Res. 14, 5252–5262 (2015).
Schweitzer, M. H., Wittmeyer, J. L. & Horner, J. R. Gender-specific reproductive tissue in ratites and Tyrannosaurus rex. Science 308, 1456–1460 (2005).
Pan, Y., Wang, Y., Sha, J. & Liao, H. Exceptional preservation of clam shrimp (Branchiopoda, Eucrustacea) eggs from the Early Cretaceous Jehol Biota and implications for paleoecology and taphonomy. J. Paleontol. 89, 369–376 (2015).
Gabbott, S., Norry, M., Aldridge, R. & Theron, J. Preservation of fossils in clay minerals; a unique example from the Upper Ordovician Soom Shale, South Africa. Proc. Yorks. Geol. Soc. 53, 237–244 (2001).
Martin, D., Briggs, D. E. & Parkes, R. J. Experimental attachment of sediment particles to invertebrate eggs and the preservation of soft-bodied fossils. J. Geol. Soc. 161, 735–738 (2004).
Pan, Y., Sha, J. & Fuersich, F. T. A model for organic fossilization of the Early Cretaceous Jehol Lagerstätte based on the taphonomy of “Ephemeropsis trisetalis”. Palaios 29, 363–377 (2014).
Wang, B., Zhao, F., Zhang, H., Fang, Y. & Zheng, D. Widespread pyritization of insects in the Early Cretaceous Jehol Biota. Palaios 27, 707–711 (2012).
O’Connor, J. K. The trophic habits of early birds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 513, 178–195 (2019).
O’Connor, J. K. & Zhou, Z. The evolution of the modern avian digestive system: insights from paravian fossils from the Yanliao and Jehol biotas. Palaeontology 63, 13–27 (2020).
Wilson, A. Principles of Plant Cell Biology (Callisto Reference, 2017).
Yeats, T. H. & Rose, J. K. The formation and function of plant cuticles. Plant Physiol. 163, 5–20 (2013).
Rodin, R. & Kapil, R. Comparative anatomy of the seed coats of Gnetum and their probable evolution. Am. J. Bot. 56, 420–431 (1969).
Wu, Y., You, H.-L. & Li, X.-Q. Dinosaur-associated Poaceae epidermis and phytoliths from the Early Cretaceous of China. Natl Sci. Rev. 5, 721–727 (2017).
Pan, Y. et al. Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis. Proc. Natl Acad. Sci. USA 113, E7900–E7907 (2016).
Acknowledgements
We thank Zhang Shukang for making ground-sections of STM10–12, Hua Xiang for SEM and EDS access. We are indebted to Jasmina Wiemann for interesting discussions about molecular preservation. For various discussions on many subjects, we thank Zhou Zhonghe, Pan Yanhong, Mary Schweitzer, Else-Marie Friis, Benjamin Bomfleur, Mihai Tomescu, Karen Chin, Wu Yan, Guan Ying, and Barbara Kremer. We thank Wu Yan for looking for potential phytoliths. This work was supported by the Chinese Academy of Sciences-President’s International Fellowship Initiative (CAS-PIFI) program and the National Natural Science Foundation of China (41688103).
Author information
Authors and Affiliations
Contributions
A.M.B. and J.O’C. designed the study. A.M.B., J.O’C., Z.L., Q.W., T.Z. collected the data. A.M.B., J.O’C., Z.L., M.A.M.M. interpreted and analyzed the data. M.W. and X.Z. contributed reagents/materials/analysis tools. A.M.B. and J.O’C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bailleul, A.M., O’Connor, J., Li, Z. et al. Confirmation of ovarian follicles in an enantiornithine (Aves) from the Jehol biota using soft tissue analyses. Commun Biol 3, 399 (2020). https://doi.org/10.1038/s42003-020-01131-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-01131-9
This article is cited by
-
Nuclear preservation in the cartilage of the Jehol dinosaur Caudipteryx
Communications Biology (2021)
-
Reanalysis of putative ovarian follicles suggests that Early Cretaceous birds were feeding not breeding
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.