Abstract
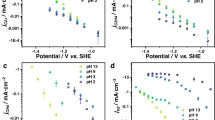
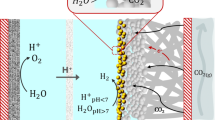
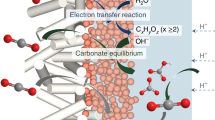
Product selectivity differences between the electroreduction of CO2 and CO under practical current densities are a widely encountered phenomenon that is rarely emphasized or investigated in the field. In this Perspective we have systematically gathered and structured data pertaining to CO2 and CO electroreduction to underscore the disparities in multi-carbon product formation. In addition, we propose that contributions of the microenvironment and a change in the local pH caused by the formation of carbonate/bicarbonate ions are among the most viable reasons behind such differences in electrochemical performance. Investigating the in situ microenvironment during the electrolysis of CO2 compared with CO will deepen the mechanistic understanding of different reaction pathways and reveal fundamental insights that may facilitate catalyst design and device-engineering strategies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the find of this study are available from the corresponding author upon reasonable request.
References
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Fan, L., Xia, C., Zhu, P., Lu, Y. & Wang, H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11, 3633 (2020).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 4614 (2018).
Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Nat. Catal. 2, 251–258 (2019).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Li, Y. C. et al. Binding site diversity rromotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Ahmad, T. et al. Electrochemical CO2 reduction to C2+ products using Cu-based electrocatalysts: a review. Nano Res. Energy 1, e9120021 (2022).
Garza, A. J., Bell, A. T. & Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 8, 1490–1499 (2018).
Xiao, H., Cheng, T. & Goddard, W. A. Atomistic mechanisms underlying selectivities in C1 and C2 products from electrochemical reduction of CO on Cu(111). J. Am. Chem. Soc. 139, 130–136 (2017).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Overa, S., Feric, T. G., Park, A.-H. A. & Jiao, F. Tandem and hybrid processes for carbon dioxide utilization. Joule 5, 8–13 (2021).
Romero Cuellar, N. S. et al. Two-step electrochemical reduction of CO2 towards multi-carbon products at high current densities. J. CO2 Util. 36, 263–275 (2020).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Wang, L. et al. Electrochemically converting carbon monoxide to liquid fuels by directing selectivity with electrode surface area. Nat. Catal. 2, 702–708 (2019).
Larrazábal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Song, Y., Zhang, X., Xie, K., Wang, G. & Bao, X. High‐temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. Adv. Mater. 31, 1902033 (2019).
Zhang, L., Hu, S., Zhu, X. & Yang, W. Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 26, 593–601 (2017).
Sisler, J. et al. Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett. 6, 997–1002 (2021).
Lu, X. et al. In situ observation of the pH gradient near the gas diffusion electrode of CO2 reduction in alkaline electrolyte. J. Am. Chem. Soc. 142, 15438–15444 (2020).
Lin, M., Han, L., Singh, M. R. & Xiang, C. An experimental- and simulation-based evaluation of the CO2 utilization efficiency of aqueous-based electrochemical CO2 reduction reactors with ion-selective membranes. ACS Appl. Energy Mater. 2, 5843–5850 (2019).
Ma, M., Kim, S., Chorkendorff, I. & Seger, B. Role of ion-selective membranes in the carbon balance for CO2 electroreduction via gas diffusion electrode reactor designs. Chem. Sci. 11, 8854–8861 (2020).
Garg, S. et al. How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers. Energy Environ. Sci. 16, 1631–1643 (2023).
Moss, A. B. et al. In operando investigations of oscillatory water and carbonate effects in MEA-based CO2 electrolysis devices. Joule 7, 350–365 (2023).
Lees, E. W., Mowbray, B. A. W., Parlane, F. G. L. & Berlinguette, C. P. Gas diffusion electrodes and membranes for CO2 reduction electrolysers. Nat. Rev. Mater. 7, 55–64 (2022).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Kim, J. Y. ‘Timothy’ et al. Recovering carbon losses in CO2 electrolysis using a solid electrolyte reactor. Nat. Catal. 5, 288–299 (2022).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Chen, X. et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 4, 20–27 (2021).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Zhang, X. et al. Selective and high current CO2 electro-reduction to multicarbon products in near-neutral KCl electrolytes. J. Am. Chem. Soc. 143, 3245–3255 (2021).
Zhu, P. et al. Direct and continuous generation of pure acetic acid solutions via electrocatalytic carbon monoxide reduction. Proc. Natl Acad. Sci. USA 118, e2010868118 (2021).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Chen, R. et al. Highly selective production of ethylene by the electroreduction of carbon monoxide. Angew. Chem. Int. Ed. 59, 154–160 (2020).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Ji, Y. et al. Selective CO-to-acetate electroproduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Zhang, J. et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. Nat. Commun. 14, 1298 (2023).
Wu, Z.-Z. et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 144, 259–269 (2022).
Wei, X. et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces. ACS Catal. 10, 4103–4111 (2020).
Wei, P. et al. Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis. Nat. Nanotechnol. 18, 299–306 (2023).
Rong, W. et al. Size-dependent activity and selectivity of atomic-level copper nanoclusters during CO/CO2 electroreduction. Angew. Chem. Int. Ed. 60, 466–472 (2021).
Jin, J. et al. Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction. Nature 617, 724–729 (2023).
Ji, Y., Yang, C., Qian, L., Zhang, L. & Zheng, G. Promoting electrocatalytic carbon monoxide reduction to ethylene on copper–polypyrrole interface. J. Colloid Interface Sci. 600, 847–853 (2021).
Guo, S. et al. Promoting electrolysis of carbon monoxide toward acetate and 1-propanol in flow electrolyzer. ACS Energy Lett. 8, 935–942 (2023).
Ma, M. et al. Local reaction environment for selective electroreduction of carbon monoxide. Energy Environ. Sci. 15, 2470–2478 (2022).
Henckel, D. A. et al. Potential dependence of the local pH in a CO2 reduction electrolyzer. ACS Catal. 11, 255–263 (2021).
Gurudayal et al. Sequential cascade electrocatalytic conversion of carbon dioxide to C–C coupled products. ACS Appl. Energy Mater. 2, 4551–4559 (2019).
Watkins, N. B. et al. Hydrodynamics change Tafel slopes in electrochemical CO2 reduction on copper. ACS Energy Lett. 8, 2185–2192 (2023).
Ma, W. et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 50, 12897–12914 (2021).
Jackson, M. N., Jung, O., Lamotte, H. C. & Surendranath, Y. Donor-dependent promotion of interfacial proton-coupled electron transfer in aqueous electrocatalysis. ACS Catal. 9, 3737–3743 (2019).
Kastlunger, G. et al. Using pH dependence to understand mechanisms in electrochemical CO reduction. ACS Catal. 12, 4344–4357 (2022).
Heenen, H. et al. The mechanism for acetate formation in electrochemical CO(2) reduction on Cu: selectivity with potential, pH, and nanostructuring. Energy Environ. Sci. 15, 3978–3990 (2022).
Wang, L. et al. Electrochemical carbon monoxide reduction on polycrystalline copper: effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 8, 7445–7454 (2018).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Warburton, R. E., Soudackov, A. V. & Hammes-Schiffer, S. Theoretical modeling of electrochemical proton-coupled electron transfer. Chem. Rev. 122, 10599–10650 (2022).
Dattila, F., Seemakurthi, R. R., Zhou, Y. & López, N. Modeling operando electrochemical CO2 reduction. Chem. Rev. 122, 11085–11130 (2022).
Zhang, T. et al. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat. Catal. 5, 202–211 (2022).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Marcandalli, G., Monteiro, M. C. O., Goyal, A. & Koper, M. T. M. Electrolyte effects on CO2 electrochemical reduction to CO. Acc. Chem. Res. 55, 1900–1911 (2022).
Acknowledgements
We acknowledge the support from the National Science Foundation grant no. 2029442, the Welch Foundation Research grant (C-2051-20230405) and the David and Lucile Packard Foundation (grant no. 2020-71371).
Author information
Authors and Affiliations
Contributions
J.Y.T.K., C.S., T.S. and H.W. conceived the idea of the Perspective. J.Y.T.K., C.S. and S.H. gathered the data and conducted the literature review. J.Y.T.K. and C.S. draughted the manuscript and created all of the figures. T.S. and H.W. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Carlos Morales-Guio and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J.Y.‘., Sellers, C., Hao, S. et al. Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions. Nat Catal 6, 1115–1124 (2023). https://doi.org/10.1038/s41929-023-01082-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01082-4