Abstract
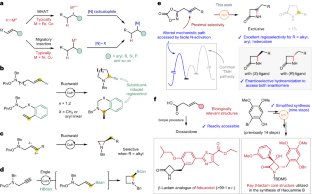
Synthetic methods for constructing enantioenriched β-lactams are highly valuable given their ubiquity in bioactive compounds, most notably in antibiotics such as penicillins and carbapenems. Intramolecular hydroamidation of β,γ-unsaturated amides would provide a convenient means to reach this alluring chemical space, yet it remains limited due to the regioselectivity issue arising from the difficulty associated with the formation of strained four-membered rings. Here we describe a NiH-catalysed strategy that addresses this challenge through the use of readily accessible alkenyl dioxazolone derivatives. The reaction transcends the conventional NiH operation mode via a transposed mechanism initiated by N-activation, thus allowing for proximal C–N bond formation with excellent regioselectivity, regardless of the electronic properties of substituents. This mechanistic platform is also highly effective for the enantioselective intramolecular hydroamidation of alkenes to enable a convenient access to enantioenriched β-lactams.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The X-ray crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC2236294 (2), CCDC2236394 (15), CCDC2236312 (17) and CCDC2236313 ((R)-22). These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the authors upon reasonable request.
References
Jordan, A. J., Lalic, G. & Sadighi, J. P. Coinage metal hydrides: synthesis, characterization, and reactivity. Chem. Rev. 116, 8318–8372 (2016).
Eberhardt, N. A. & Guan, H. Nickel hydride complexes. Chem. Rev. 116, 8373–8426 (2016).
Hu, Y., Shaw, A. P., Estes, D. P. & Norton, J. R. Transition-metal hydride radical cations. Chem. Rev. 116, 8427–8462 (2016).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Green, S. A. et al. The high chemofidelity of metal-catalyzed hydrogen atom transfer. Acc. Chem. Res. 51, 2628–2640 (2018).
Chen, J., Guo, J. & Lu, Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition metal catalysis. Chin. J. Chem. 36, 1075–1109 (2018).
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley‐VCH, 2007).
Hirano, K. & Miura, M. Hydroamination, aminoboration, and carboamination with electrophilic amination reagents: Umpolung-enabled regio- and stereoselective synthesis of N-containing molecules from alkenes and alkynes. J. Am. Chem. Soc. 144, 648–661 (2022).
Miki, Y., Hirano, K., Satoh, T. & Miura, M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Ed. 52, 10830–10834 (2013).
Zhu, S., Niljianskul, N. & Buchwald, S. L. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 135, 15746–15749 (2013).
Müller, T. E., Hultzsch, K. C., Yus, M., Foubelo, F. & Tada, M. Hydroamination: direct addition of amines to alkenes and alkynes. Chem. Rev. 108, 3795–3892 (2008).
Huang, L., Arndt, M., Gooßen, K., Heydt, H. & Gooßen, L. J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 115, 2596–2697 (2015).
Liu, R. Y. & Buchwald, S. L. CuH-catalyzed olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 53, 1229–1243 (2020).
Gui, J. et al. Practical olefin hydroamination with nitroarenes. Science 348, 886–891 (2015).
Song, H., Yang, Z., Tung, C.-H. & Wang, W. Iron-catalyzed reductive coupling of nitroarenes with olefins: Intermediate of iron–nitroso complex. ACS Catal. 10, 276–281 (2020).
Chen, J., Shen, X. & Lu, Z. Cobalt-catalyzed Markovnikov selective sequential hydrogenation/hydrohydrazidation of aliphatic terminal alkynes. J. Am. Chem. Soc. 142, 14455–14460 (2020).
Shen, X. et al. Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes. Nat. Commun. 11, 783 (2020).
Wang, Y., He, Y. & Zhu, S. NiH-catalyzed functionalization of remote and proximal olefins: new reactions and innovative strategies. Acc. Chem. Res. 55, 3519–3536 (2022).
Dai, X.-J., Engl, O. D., León, T. & Buchwald, S. L. Catalytic asymmetric synthesis of α-arylpyrrolidines and benzo-fused nitrogen heterocycles. Angew. Chem. Int. Ed. 58, 3407–3411 (2019).
Xi, Y., Butcher, T. W., Zhang, J. & Hartwig, J. F. Regioselective, asymmetric formal hydroamination of unactivated internal alkenes. Angew. Chem. Int. Ed. 55, 776–780 (2016).
Ichikawa, S., Dai, X.-J. & Buchwald, S. L. Regio- and enantioselective synthesis of 1,2-diamine derivatives by copper-catalyzed hydroamination. Org. Lett. 21, 4370–4373 (2019).
Shigehisa, H. et al. Catalytic hydroamination of unactivated olefins using a Co catalyst for complex molecule synthesis. J. Am. Chem. Soc. 136, 13534–13537 (2014).
Osato, A., Fujihara, T. & Shigehisa, H. Constructing four-membered heterocycles by cycloisomerization. ACS Catal. 13, 4101–4110 (2023).
Wang, H., Yang, J. C. & Buchwald, S. L. CuH-catalyzed regioselective intramolecular hydroamination for the synthesis of alkyl-substituted chiral aziridines. J. Am. Chem. Soc. 139, 8428–8431 (2017).
Gao, D.-W. et al. Cascade CuH-catalysed conversion of alkynes into enantioenriched 1,1-disubstituted products. Nat. Catal. 3, 23–29 (2020).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Banik, B. K. Beta-Lactams: Novel Synthetic Pathways and Applications (Springer Cham, 2017).
Pratley, C., Fenner, S. & Murphy, J. A. Nitrogen-centered radicals in functionalization of sp2 systems: generation, reactivity, and applications in synthesis. Chem. Rev. 122, 8181–8260 (2022).
van Vliet, K. M. & de Bruin, B. Dioxazolones: Stable substrates for the catalytic transfer of acyl nitrenes. ACS Catal. 10, 4751–4769 (2020).
Hong, S. Y., Hwang, Y., Lee, M. & Chang, S. Mechanism-guided development of transition-metal-catalyzed C–N bond-forming reactions using dioxazolones as the versatile amidating source. Acc. Chem. Res. 54, 2683–2700 (2021).
Lyu, X., Zhang, J., Kim, D., Seo, S. & Chang, S. Merging NiH catalysis and inner-sphere metal-nitrenoid transfer for hydroamidation of alkynes. J. Am. Chem. Soc. 143, 5867–5877 (2021).
Choi, H., Lyu, X., Kim, D., Seo, S. & Chang, S. Endo-selective intramolecular alkyne hydroamidation enabled by NiH catalysis incorporating alkenylnickel isomerization. J. Am. Chem. Soc. 144, 10064–10074 (2022).
Meng, L., Yang, J., Duan, M., Wang, Y. & Zhu, S. Facile synthesis of chiral arylamines, alkylamines and amides by enantioselective NiH-catalyzed hydroamination. Angew. Chem. Int. Ed. 60, 23584–23589 (2021).
Zhang, Y., Qiao, D., Duan, M., Wang, Y. & Zhu, S. Enantioselective synthesis of α-aminoboronates by NiH-catalysed asymmetric hydroamidation of alkenyl boronates. Nat. Commun. 13, 5630 (2022).
Li, Y. et al. A general strategy for the synthesis of α-trifluoromethyl- and α-perfluoroalkyl-β-lactams via palladium-catalyzed carbonylation. Chem. Sci. 12, 10467–10473 (2021).
Fischer, D. M., Balkenhohl, M. & Carreira, E. M. Cobalt-catalyzed cyclization of unsaturated N-acyl sulfonamides: a diverted Mukaiyama hydration reaction. JACS Au 2, 1071–1077 (2022).
Marson, C. M. & Fallah, A. Preparation of γ- and δ-lactams by ring closure of β,γ-unsaturated amides using trifluoromethanesulfonic acid. Tetrahedron Lett. 35, 293–296 (1994).
Qi, C., Hasenmaile, F., Gandon, V. & Lebœuf, D. Calcium(II)-catalyzed intra- and intermolecular hydroamidation of unactivated alkenes in hexafluoroisopropanol. ACS Catal. 8, 1734–1739 (2018).
Wang, H. et al. Regioselective intramolecular Markovnikov and anti-Markovnikov hydrofunctionalization of alkenes via photoredox catalysis. Chem. Commun. 55, 11426–11429 (2019).
Jeon, J., Lee, C., Seo, H. & Hong, S. NiH-catalyzed proximal-selective hydroamination of unactivated alkenes. J. Am. Chem. Soc. 142, 20470–20480 (2020).
Lee, C., Seo, H., Jeon, J. & Hong, S. γ-Selective C(sp3)–H amination via controlled migratory hydroamination. Nat. Commun. 12, 5657 (2021).
Du, B., Ouyang, Y., Chen, Q. & Yu, W.-Y. Thioether-directed NiH-catalyzed remote γ-C(sp3)–H hydroamidation of alkenes by 1,4,2-dioxazol-5-ones. J. Am. Chem. Soc. 143, 14962–14968 (2021).
Lee, C., Kang, H.-J., Seo, H. & Hong, S. Nickel-catalyzed regio- and enantioselective hydroamination of unactivated alkenes using carbonyl directing groups. J. Am. Chem. Soc. 144, 9091–9100 (2022).
Du, B. et al. NiH-catalyzed anti-Markovnikov hydroamidation of unactivated alkenes with 1,4,2-dioxazol-5-ones for the direct synthesis of N-alkyl amides. Commun. Chem. 5, 176 (2022).
Hong, S. Y. & Chang, S. Stereodefined access to lactams via olefin difunctionalization: iridium nitrenoids as a motif of LUMO-controlled dipoles. J. Am. Chem. Soc. 141, 10399–10408 (2019).
Hong, S. Y., Kim, D. & Chang, S. Catalytic access to carbocation intermediates via nitrenoid transfer leading to allylic lactams. Nat. Catal. 4, 79–88 (2021).
Kim, S., Kim, D., Hong, S. Y. & Chang, S. Tuning orbital symmetry of iridium nitrenoid enables catalytic diastereo- and enantioselective alkene difunctionalizations. J. Am. Chem. Soc. 143, 3993–4004 (2021).
Kweon, J., Kim, D., Kang, S. & Chang, S. Access to β-lactams via iron-catalyzed olefin oxyamidation enabled by the π-accepting phthalocyanine ligand. J. Am. Chem. Soc. 144, 1872–1880 (2022).
Polse, J. L., Andersen, R. A. & Bergman, R. G. Reactivity of a terminal Ti(IV) imido complex toward alkenes and alkynes: cycloaddition vs C–H activation. J. Am. Chem. Soc. 120, 13405–13414 (1998).
Day, C. S. et al. Elucidating electron-transfer events in polypyridine nickel complexes for reductive coupling reactions. Nat. Catal. 6, 244–253 (2023).
Brandi, A., Cicchi, S. & Cordero, F. M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 108, 3988–4035 (2008).
Hodous, B. L. & Fu, G. C. Enantioselective Staudinger aynthesis of β-lactams catalyzed by a planar-chiral nucleophile. J. Am. Chem. Soc. 124, 1578–1579 (2002).
Khangarot, R. K. & Kaliappan, K. P. Kinugasa reaction: a direct one-pot route to highly functionalized β-lactams. Eur. J. Org. Chem. 2013, 7664–7677 (2013).
Ye, Y. et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination. Nat. Chem. 15, 206–212 (2022).
Fujioka, H., Yamanaka, T., Matsunaga, N., Fuji, M. & Kita, Y. Asymmetric synthesis of carbapenems using chiral acetals: synthesis of a key intermediate to (+)-PS-5. Synlett 1992, 35–36 (1992).
Starcevic, S., Mrak, P. & Kopitar, G. An enzymatic route for the preparation of chiral γ-aryl-β-aminobutyric acid derivatives. WO2014096375 (2014).
Momoi, Y. et al. Total synthesis of (−)-Haouamine B pentaacetate and structural revision of Haouamine B. Angew. Chem. Int. Ed. 53, 13215–13219 (2014).
Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R010-Y2 (S.S.) and IBS-R010-D1 (S.C.)) in South Korea. S.S. also acknowledges support from the DGIST Start-up Fund Program of the Ministry of Science and ICT (2023040012). Computational works for this research were performed on the High Performance Computing Resources in the IBS Research Solution Center.
Author information
Authors and Affiliations
Contributions
S.S. and S.C. conceived and designed the project. X.L. optimized the reaction conditions for asymmetric hydroamination, and performed and analysed the experiments for the reaction scope and mechanistic investigations. X.L. and C.S. carried out experiments for the synthetic applications. T.F. contributed to the initial findings and reaction optimization of the standard hydroamidation reaction. D.K. performed the X-ray crystallographic analysis. H.J. carried out the DFT calculations. S.S and S.C. organized the research and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Cristina Trujillo and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–15, Figs. 1–22 and references.
Supplementary Data 1
Crystallographic data for compound 2.
Supplementary Data 2
Crystallographic data for compound 15.
Supplementary Data 3
Crystallographic data for compound 17.
Supplementary Data 4
Crystallographic data for compound (R)-22.
Supplementary Data 5
Cartesian coordinates of DFT-optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, X., Seo, C., Jung, H. et al. Intramolecular hydroamidation of alkenes enabling asymmetric synthesis of β-lactams via transposed NiH catalysis. Nat Catal 6, 784–795 (2023). https://doi.org/10.1038/s41929-023-01014-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01014-2



