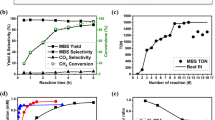
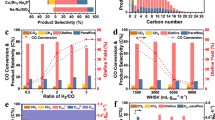
Abstract
An efficient route for selective methane functionalization to liquid products, such as methanol, without intermediate syngas production is an integral part of the movement toward greener chemical and fuel production from currently underutilized resource streams. This challenging chemistry has motivated grand scientific efforts in the study of C–H activation and highly selective active site motifs, yet substantial limitations inhibit the translation of these concepts into practical processes. Here we assess recent developments in methane partial oxidation from thermochemical, photochemical, electrochemical, and non-thermal plasma literature published within the past five years using quantitative performance indicators. Ultimately, the field of methane valorization is unlikely to surpass limiting barriers on its current trajectory. Comprehensive design and innovation that target the improvement of multiple metrics (yield, productivity, product concentration) simultaneously with the incorporation of product protection schemes are paramount, as outlined in a roadmap for concepts with high potential for implementation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vogel, F. Chasing after methane’s ultra-emitters. Science 375, 490–491 (2022).
Ravi, M., Ranocchiari, M. & van Bokhoven, J. A. The direct catalytic oxidation of methane to methanol—a critical assessment. Angew. Chem. Int. Ed. 56, 16464–16483 (2017).
Lawton, T. J. & Rosenzweig, A. C. Methane-oxidizing enzymes: an upstream problem in biological gas-to-liquids conversion. J. Am. Chem. Soc. 138, 9327–9340 (2016).
Wang, V. C. C. et al. Alkane oxidation: methane monooxygenases, related enzymes, and their biomimetics. Chem. Rev. 117, 8574–8621 (2017).
Latimer, A. A., Kakekhani, A., Kulkarni, A. R. & Norskov, J. K. Direct methane to methanol: the selectivity-conversion limit and design strategies. ACS Catal. 8, 6894–6907 (2018).
Ahlquist, M., Nielsen, R. J., Periana, R. A. & Goddard Iii, W. A. Product protection, the key to developing high performance methane selective oxidation catalysts. J. Am. Chem. Soc. 131, 17110–17115 (2009).
Mlekodaj, K., Lemishka, M., Sklenak, S., Dedecek, J. & Tabor, E. Dioxygen splitting at room temperature over distant binuclear transition metal centers in zeolites for direct oxidation of methane to methanol. Chem. Commun. 57, 3472–3475 (2021).
Tabor, E. et al. Dioxygen dissociation over man-made system at room temperature to form the active alpha-oxygen for methane oxidation. Sci. Adv. 6, eaaz9776 (2020).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017).
Lee, S. H., Kang, J. K. & Park, E. D. Continuous methanol synthesis directly from methane and steam over Cu(II)-exchanged mordenite. Korean J. Chem. Eng. 35, 2145–2149 (2018).
Jovanovic, Z. R. et al. Oxidation of methane to methanol over Cu-exchanged zeolites: Scientia gratia scientiae or paradigm shift in natural gas valorization? J. Catal. 385, 238–245 (2020).
Jocz, J. N., Medford, A. J. & Sievers, C. Thermodynamic limitations of the catalyst design space for methanol production from methane. ChemCatChem 11, 593–600 (2019).
Artsiusheuski, M. A., Verel, R., van Bokhoven, J. A. & Sushkevich, V. L. Methane transformation over copper-exchanged zeolites: from partial oxidation to C–C coupling and formation of hydrocarbons. ACS Catal. 11, 12543–12556 (2021).
Sushkevich, V. L. & van Bokhoven, J. A. Effect of Brønsted acid sites on the direct conversion of methane into methanol over copper-exchanged mordenite. Catal. Sci. Technol. 8, 4141–4150 (2018).
Dyballa, M. et al. Zeolite surface methoxy groups as key intermediates in the stepwise conversion of methane to methanol. ChemCatChem 11, 5022–5026 (2019).
Yamasaki, T. et al. Low-temperature activation of methane with nitric oxide and formation of hydrogen cyanide over an alumina-supported platinum catalyst. ACS Catal. 11, 14660–14668 (2021).
Pappas, D. K. et al. Methane to methanol: structure activity relationships for Cu-CHA. J. Am. Chem. Soc. 139, 14961–14975 (2017).
Narsimhan, K., Iyoki, K., Dinh, K. & Roman-Leshkov, Y. Catalytic oxidation of methane into methanol over copper-exchanged zeolites with oxygen at low temperature. ACS Cent. Sci. 2, 424–429 (2016). Demonstration of the catalytic conversion of methane to methanol in a gas–solid mode enabled by co-feeding water along with methane and oxygen over copper-exchanged zeolites.
Hirayama, A. et al. Catalytic oxidation of methane to methanol over Cu-CHA with molecular oxygen. Catal. Sci. Technol. 11, 6217–6224 (2021).
Vargheese, V., Kobayashi, Y. & Oyama, S. T. The direct partial oxidation of methane to dimethyl ether over Pt/Y2O3catalysts using an NO/O2 shuttle. Angew. Chem. Int. Ed. 59, 16644–16650 (2020).
Vargheese, V. et al. The direct molecular oxygen partial oxidation of CH4 to dimethyl ether without methanol formation over a Pt/Y2O3 catalyst using an NO/NO2 oxygen atom shuttle. J. Catal. 389, 352–365 (2020).
Ghampson, I. T. et al. Methane selective oxidation on metal oxide catalysts at low temperatures with O2 using an NO/NO2 oxygen atom shuttle. J. Catal. 408, 401–412 (2022).
Dinh, K. T., Sullivan, M. M., Serna, P., Meyer, R. J. & Roman-Leshkov, Y. Breaking the selectivity-conversion limit of partial methane oxidation with tandem heterogeneous catalysts. ACS Catal. 11, 9262–9270 (2021). A concept for the tandem catalytic conversion of methane to methanol with subsequent alkylation of benzene to toluene using a mixture of small-pore copper-exchanged zeolites and larger-pore zeolite.
Lange, J. P. In Sustainable Strategies for the Upgrading of Natural Gas: Fundamentals, Challenges, and Opportunities (eds. Derouane, E. G. et al.) 51–83 (NATO Science Series II: Mathematics, Physics and Chemistry, Vol. 191, 2005).
Periana, R. A. et al. Perspectives on some challenges and approaches for developing the next generation of selective, low temperature, oxidation catalysts for alkane hydroxylation based on the CH activation reaction. J. Mol. Catal. A 220, 7–25 (2004).
Bunting, R. J., Rice, P. S., Thompson, J. & Hu, P. Investigating the innate selectivity issues of methane to methanol: consideration of an aqueous environment. Chem. Sci. 12, 4443–4449 (2021).
Jin, Z. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 367, 193–197 (2020). A nanoreactor concept involving zeolite-encapsulated AuPd nanoparticles with a hydrophobic coating generating hydrogen peroxide in situ near methane activation sites, allowing high methanol selectivity at larger extents of methane conversion.
Periana, R. A. et al. Amercury-catalyzed, high-yield system for the oxidation of methane to methanol. Science 259, 340–343 (1993).
Periana, R. A. et al. Platinum catalysts for the high-yield oxidation of methane to a methanol derivative. Science 280, 560–564 (1998).
Michalkiewicz, B. Methane conversion to methanol in condensed phase. Kinet. Catal. 44, 801–805 (2003).
Gang, X., Zhu, Y. M., Birch, H., Hjuler, H. A. & Bjerrum, N. J. Iodine as catalyst for the direct oxidation of methane to methyl sulfates in oleum. Appl Catal. A 261, 91–98 (2004).
Zimmermann, T., Soorholtz, M., Bilke, M. & Schüth, F. Selective methane oxidation catalyzed by platinum salts in oleum at turnover frequencies of large-scale industrial processes. J. Am. Chem. Soc. 138, 12395–12400 (2016).
Conley, B. L. et al. Design and study of homogeneous catalysts for the selective, low temperature oxidation of hydrocarbons. J. Mol. Catal. A 251, 8–23 (2006).
Blankenship, A. N., Ravi, M. & van Bokhoven, J. A. Esterification product protection strategies for direct and selective methane conversion. Chimia 75, 305–310 (2021).
Noceti, R. P., Taylor, C. E. & D’Este, J. R. Photocatalytic conversion of methane. Catal. Today 33, 199–204 (1997).
Li, X. Y., Wang, C. & Tang, J. W. Methane transformation by photocatalysis. Nat. Rev. Mater. 7, 617–632 (2022).
Li, Q., Ouyang, Y. X., Li, H. L., Wang, L. B. & Zeng, J. Photocatalytic conversion of methane: recent advancements and prospects. Angew. Chem. Int. Ed. 61, e202108069 (2022).
Ohkubo, K. & Hirose, K. Light-driven C–H oxygenation of methane into methanol and formic acid by molecular oxygen using a perfluorinated solvent. Angew. Chem. Int Ed. 57, 2126–2129 (2018). The photochemical conversion of methane with a high product yield enabled by a biphasic solvent system, in which the product is sequestered into an oxidant-poor phase to prevent overoxidation.
Liebov, N. S. et al. Selective photo-oxygenation of light alkanes using iodine oxides and chloride. ChemCatChem 11, 5045–5054 (2019).
Sun, H. L. et al. Ultra-stable molecular interface SiW12Ox/TiO2 catalyst derived from keggin-type polyoxometalates for photocatalytic conversion of methane to oxygenates. ChemCatChem 14, e202200001 (2022).
Wu, X. Y. et al. Noble-metal-free dye-sensitized selective oxidation of methane to methanol with green light (550 nm). Nano Res. 14, 4584–4590 (2021).
An, B. et al. Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site. Nat. Mater. 21, 932–938 (2022). A flow reaction concept for photocatalytic methane conversion using iron-containing MOFs with relatively high product concentrations and activity using a molecular oxygen oxidant.
Feng, G. H. et al. Solar driven efficient direct conversion of methane to multicarbon oxygenates. J. Mater. Chem. A 10, 7856–7868 (2022).
Luo, L. et al. Binary Au-Cu reaction sites decorated ZnO for selective methane oxidation to C1 oxygenates with nearly 100% selectivity at room temperature. J. Am. Chem. Soc. 144, 740–750 (2022).
Sakata, Y., Hayashi, T., Yasunaga, R., Yanaga, N. & Imamura, H. Remarkably high apparent quantum yield of the overall photocatalytic H2O splitting achieved by utilizing Zn ion added Ga2O3 prepared using dilute CaCl2 solution. Chem. Commun. 51, 12935–12938 (2015).
Takata, T. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020).
Zhao, Y. et al. A hydrogen farm strategy for scalable solar hydrogen production with particulate photocatalysts. Angew. Chem. Int. Ed. 59, 9653–9658 (2020).
Han, B., Wei, W., Li, M. J., Sun, K. & Hu, Y. H. A thermo-photo hybrid process for steam reforming of methane: highly efficient visible light photocatalysis. Chem. Commun. 55, 7816–7819 (2019).
Lopez-Martin, A., Caballero, A. & Colon, G. Photochemical methane partial oxidation to methanol assisted by H2O2. J. Photochem. Photobiol. A 349, 216–223 (2017).
Indarto, A. A review of direct methane conversion to methano by dielectric barrier discharge. IEEE Trans. Dielectr. Electr. Insul. 15, 1038–1043 (2008).
Kim, H. H., Teramoto, Y., Ogata, A., Takagi, H. & Nanba, T. Plasma catalysis for environmental treatment and energy applications. Plasma Chem. Plasma Process. 36, 45–72 (2016).
Puliyalil, H., Jurkovic, D. L., Dasireddy, V. D. B. C. & Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 8, 27481–27508 (2018).
Bogaerts, A. & Neyts, E. C. Plasma technology: an emerging technology for energy storage. ACS Energy Lett. 3, 1013–1027 (2018).
Ollegott, K., Wirth, P., Oberste-Beulmann, C., Awakowicz, P. & Muhler, M. Fundamental properties and applications of dielectric barrier discharges in plasma-catalytic processes at atmospheric pressure. Chem. Ing. Tech. 92, 1542–1558 (2020).
Zhang, Y. R., Neyts, E. C. & Bogaerts, A. Influence of the material dielectric constant on plasma generation inside catalyst pores. J. Phys. Chem. C 120, 25923–25934 (2016).
Wang, W. Z., Kim, H. H., Van Laer, K. & Bogaerts, A. Streamer propagation in a packed bed plasma reactor for plasma catalysis applications. Chem. Eng. J. 334, 2467–2479 (2018).
Loenders, B., Engelmann, Y. & Bogaerts, A. Plasma-catalytic partial oxidation of methane on Pt(111): a microkinetic study on the role of different plasma species. J. Phys. Chem. C 125, 2966–2983 (2021).
Jiang, J. K. & Bruggeman, P. J. Investigation of the mechanisms underpinning plasma-catalyst interaction for the conversion of methane to oxygenates. Plasma Chem. Plasma Process. 42, 689–707 (2022).
Yan, C. et al. Recent advances in plasma catalysis. J. Phys. Chem. C 126, 9611–9614 (2022).
Lee, H. & Kim, D. H. Direct methanol synthesis from methane in a plasma-catalyst hybrid system at low temperature using metal oxide-coated glass beads. Sci. Rep. 8, 9956 (2018).
Indarto, A. Partial oxidation of methane to methanol with nitrogen dioxide in dielectric barrier discharge plasma: experimental and molecular modeling. Plasma Sources Sci. Technol. 25, 025002 (2016).
Hinde, P., Demidyuk, V., Gkelios, A. & Tipton, C. Plasma catalysis: a review of the interdisciplinary challenges faced realising the potential of plasma catalysis on a commercial scale. Johnson Matthey Technol. Rev. 64, 138–147 (2020).
Fathollahi, P. et al. Selective oxidation of methane to methanol by NTP plasma: the effect of power and oxygen on conversion and selectivity. J. Electrostat. 112, 103594 (2021). Plasma-assisted conversion of methane using copper electrodes that result in better energy efficiency relative to product yield.
Larkin, D. W., Lobban, L. L. & Mallinson, R. G. The direct partial oxidation of methane to organic oxygenates using a dielectric barrier discharge reactor as a catalytic reactor analog. Catal. Today 71, 199–210 (2001).
Larkin, D. W., Zhou, L. M., Lobban, L. L. & Mallinson, R. G. Product selectivity control and organic oxygenate pathways from partial oxidation of methane in a silent electric discharge reactor. Ind. Eng. Chem. Res. 40, 5496–5506 (2001).
Nozaki, T., Hattori, A. & Okazaki, K. Partial oxidation of methane using a microscale non-equilibrium plasma reactor. Catal. Today 98, 607–616 (2004).
Nozaki, T., Agiral, A., Yuzawa, S., Gardeniers, J. G. E. H. & Okazaki, K. A single step methane conversion into synthetic fuels using microplasma reactor. Chem. Eng. J. 166, 288–293 (2011).
Okumoto, M. & Mizuno, A. Conversion of methane for higher hydrocarbon fuel synthesis using pulsed discharge plasma method. Catal. Today 71, 211–217 (2001).
Bagherzadeh Mostaghimi, A. H., Al-Attas, T. A., Kibria, M. G. & Siahrostami, S. A review on electrocatalytic oxidation of methane to oxygenates. J. Mater. Chem. A 8, 15575–15590 (2020).
Arminio-Ravelo, J. A. & Escudero-Escribano, M. Strategies toward the sustainable electrochemical oxidation of methane to methanol. Curr. Opin. Green. Sustain. Chem. 30, 100489 (2021).
Wang, Q., Kan, M., Han, Q. & Zheng, G. Electrochemical methane conversion. Small Struct. 2, 2100037 (2021).
Periana, R. A. et al. In Studies in Surface Science and Catalysis Vol. 81 (eds. Curry-Hyde, H. E. & Howe, R. F.) 533–544 (Elsevier, 1994).
Muehlhofer, M., Strassner, T. & Herrmann, W. A. New catalyst systems for the catalytic conversion of methane into methanol. Angew. Chem. Int. Ed. 41, 1745–1747 (2002).
Seki, Y., Min, J. S., Misono, M. & Mizuno, N. Reaction mechanism of oxidation of methane with hydrogen peroxide catalyzed by 11-molybdo-1-vanadophosphoric acid catalyst precursor. J. Phys. Chem. B 104, 5940–5944 (2000).
Yuan, S. et al. Conversion of methane into liquid fuels—bridging thermal catalysis with electrocatalysis. Adv. Energy Mater. 10, 2002154 (2020).
Sher Shah, M. S. A. et al. Catalytic oxidation of methane to oxygenated products: recent advancements and prospects for electrocatalytic and photocatalytic conversion at low temperatures. Adv. Sci. 7, 2001946 (2020).
Lewis, R. J. et al. The direct synthesis of H2O2 and selective oxidation of methane to methanol using HZSM-5 supported AuPd catalysts. Catal. Lett. 149, 3066–3075 (2019).
Natinsky, B. S., Lu, S., Copeland, E. D., Quintana, J. C. & Liu, C. Solution catalytic cycle of incompatible steps for ambient air oxidation of methane to methanol. ACS Cent. Sci. 5, 1584–1590 (2019). A nanowire electrode is used to spatially separate incompatible process steps in electrochemical methane conversion to prevent the active metalloradical deactivation by oxygen.
Lange, J.-P., Sushkevich, V. L., Knorpp, A. J. & van Bokhoven, J. A. Methane-to-methanol via chemical looping: economic potential and guidance for future research. Ind. Eng. Chem. Res. 58, 8674–8680 (2019).
Lange, J.-P. Catalysis for biorefineries – performance criteria for industrial operation. Catal. Sci. Technol. 6, 4759–4767 (2016).
Soucie, H., Elam, M. & Mustain, W. E. Practical assessment for at-scale electrochemical conversion of methane to methanol. ACS Energy Lett. 8, 1218–1229 (2023).
Shan, J. J., Li, M. W., Allard, L. F., Lee, S. S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605 (2017).
Lee, H. W. et al. Pt black catalyzed methane oxidation to methyl bisulfate in H2SO4-SO3. J. Catal. 374, 230–236 (2019).
Zhu, K. X. et al. Highly efficient conversion of methane to formic acid under mild conditions at ZSM-5-confined Fe-sites. Nano Energy 82, 105718 (2021).
Gorky, F., Nambo, A. & Carreon, M. L. Cold plasma-metal organic framework (MOF)-177 breathable system for atmospheric remediation. J. CO2 Util. 51, 101642 (2021).
Sarno, M., Ponticorvo, E., Funicello, N. & De Pasquale, S. Methane electrochemical oxidation at low temperature on Rh single atom/NiO/V2O5 nanocomposite. Appl. Catal. A 603, 117746 (2020).
Deng, J. et al. Ambient methane functionalization initiated by electrochemical oxidation of a vanadium (V)-oxo dimer. Nat. Commun. 11, 3686 (2020).
Acknowledgements
We acknowledge financial support from ETH Zurich, the Paul Scherrer Institut and the Swiss National Science Foundation (project 200021_178943).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Maria Carreon, Daniel Shantz and Jong Hyeok Park for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–2, Tables 1–8, Figs. 1–5 and references.
Source data
Source Data Fig. 1
Source data for performance comparison in figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blankenship, A., Artsiusheuski, M., Sushkevich, V. et al. Recent trends, current challenges and future prospects for syngas-free methane partial oxidation. Nat Catal 6, 748–762 (2023). https://doi.org/10.1038/s41929-023-01000-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01000-8