Abstract
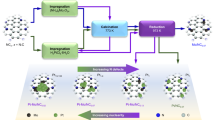
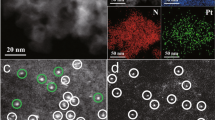
It is a great challenge to regulate the precise placement of confined metal species and assemble specific structures at the atomic level. Here we report the design and synthesis of a durable supported-metal-cluster catalyst, Pt@Ge-UTL, that features subnanometric Pt clusters encapsulated inside the extra-large pores of a stabilized UTL-type germanosilicate. Integrated differential phase contrast scanning transmission electron microscopy, in situ X-ray absorption fine structure, 19F magic-angle-spinning NMR, full-range synchrotron pair distribution function G(r) analysis and density functional theory calculations revealed that Pt clusters with an average of four atoms are firmly segregated within 14-membered-ring channels. This is achieved by selectively and directionally anchoring Pt, via Pt–O–Ge bonding, to the UTL zeolite’s unique secondary building units that feature a double-four-membered-ring (d4r) configuration and a Ge-enriched composition. The host–guest bimetallic structure (Pt4-Ge2-d4r@UTL) promotes propane dehydrogenation with high activity, high propylene selectivity and long-term stability. This research demonstrates the use of germanosilicates in the designed synthesis of high-performance propane dehydrogenation catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All the data needed to support the plots and evaluate the conclusions of this study are present within the Article and the Supplementary Information or are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Sattler, J. J. et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Xi, J. et al. Synthesis strategies, catalytic applications, and performance regulation of single-atom catalysts. Adv. Funct. Mater. 31, 2008318 (2021).
Wang, W. L. et al. Direct observation of a long-lived single-atom catalyst chiseling atomic structures in graphene. Nano Lett. 14, 450–455 (2014).
Shi, Y. et al. Single-atom catalysis in mesoporous photovoltaics: the principle of utility maximization. Adv. Mater. 26, 8147–8153 (2014).
Liu, L. et al. Evolution of isolated atoms and clusters in catalysis. Trends Chem. 2, 383–400 (2020).
Sun, G. et al. Breaking the scaling relationship via thermally stable Pt/Cu single atom alloys for catalytic dehydrogenation. Nat. Commun. 9, 4454 (2018).
Xiong, H. et al. Thermally stable and regenerable platinum–tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. 56, 8986–8991 (2017).
Goel, S. et al. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 136, 15280–15290 (2014).
Wang, N. et al. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 138, 7484–7487 (2016).
Zhang, J. et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat. Catal. 1, 540–546 (2018).
Sun, Q. et al. Synergetic effect of ultrasmall metal clusters and zeolites promoting hydrogen generation. Adv. Sci. 6, 1802350 (2019).
Sun, Q. et al. Zeolite-encaged single-atom rhodium catalysts: highly-efficient hydrogen generation and shape-selective tandem hydrogenation of nitroarenes. Angew. Chem. Int. Ed. 58, 18570–18576 (2019).
Liu, L. et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 18, 866–873 (2019).
Zhu, J. et al. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: catalytic application for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19669–19674 (2020).
Sun, Q. et al. Subnanometer bimetallic platinum–zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. 59, 19450–19459 (2020).
Wang, Y. et al. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. J. Catal. 385, 61–69 (2020).
Searles, K. et al. Highly productive propane dehydrogenation catalyst using silica-supported Ga–Pt nanoparticles generated from single-sites. J. Am. Chem. Soc. 140, 11674–11679 (2018).
Wu, Z. et al. Changes in catalytic and adsorptive properties of 2 nm Pt3Mn nanoparticles by subsurface atoms. J. Am. Chem. Soc. 140, 14870–14877 (2018).
Liu, L. et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 3, 628–638 (2020).
Uemura, Y. et al. In situ time-resolved XAFS study on the structural transformation and phase separation of Pt3Sn and PtSn alloy nanoparticles on carbon in the oxidation process. Phys. Chem. Chem. Phys. 13, 15833–15844 (2011).
Ramallo-López, J. M. et al. XPS and XAFS Pt L2,3-edge studies of dispersed metallic Pt and PtSn clusters on SiO2 obtained by organometallic synthesis: structural and electronic characteristics. J. Phys. Chem. B 107, 11441–11451 (2003).
Deng, L. et al. Elucidating strong metal–support interactions in Pt–Sn/SiO2 catalyst and its consequences for dehydrogenation of lower alkanes. J. Catal. 365, 277–291 (2018).
Xu, Z. et al. Propane dehydrogenation over Pt clusters localized at the Sn single-site in zeolite framework. ACS Catal. 10, 818–828 (2020).
Ma, Y. et al. Skeleton-Sn anchoring isolated Pt site to confine subnanometric clusters within *BEA topology. J. Catal. 397, 44–57 (2021).
Paillaud, J. et al. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings. Science 304, 990–992 (2004).
Corma, A. et al. ITQ-15: the first ultralarge pore zeolite with a bi-directional pore system formed by intersecting 14- and 12-ring channels, and its catalytic implications. Chem. Commun. 1356–1357 (2004).
Roth, W. et al. Postsynthesis transformation of three-dimensional framework into a lamellar zeolite with modifiable architecture. J. Am. Chem. Soc. 133, 6130–6133 (2011).
Roth, W. et al. A family of zeolites with controlled pore size prepared using a top-down method. Nat. Chem. 5, 628–633 (2013).
Morris, S. et al. In situ solid-state NMR and XRD studies of the ADOR process and the unusual structure of zeolite IPC-6. Nat. Chem. 9, 1012–1018 (2017).
Wheatley, P. et al. Zeolites with continuously tuneable porosity. Angew. Chem. Int. Ed. 53, 13210–13214 (2014).
Mazur, M. et al. Synthesis of ‘unfeasible’ zeolites. Nat. Chem. 8, 58–62 (2016).
Zhang, Y. et al. Encapsulation of Pt nanoparticles into IPC-2 and IPC-4 zeolites using the ADOR approach. Microporous Mesoporous Mater. 279, 364–370 (2019).
Sajad, M. et al. Direct dehydrogenation of propane over Pd nanoparticles encapsulated within IPC zeolites with tunable pore sizes. Appl. Mater. Today 29, 101644 (2022).
Li, A. et al. Encapsulating metal nanoparticles into a layered zeolite precursor with surface silanol nests enhances sintering resistance. Angew. Chem. Int. Ed. 62, e202213361 (2023).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Xu, H. et al. Post-synthesis treatment gives highly stable siliceous zeolites through the isomorphous substitution of silicon for germanium in germanosilicates. Angew. Chem. Int. Ed. 53, 1355–1359 (2014).
De La Cruz, C. et al. An exploration of the surfaces of some Pt/SiO2 catalysts using CO as an infrared spectroscopic probe. Spectrochim. Acta A 50, 271–285 (1994).
Stakheev, A. Y. et al. Electronic state and location of Pt metal clusters in KL zeolite: FTIR study of CO chemisorption. Catal. Lett. 32, 147–158 (1995).
Klünker, C. et al. CO stretching vibrations on Pt(111) and Pt(110) studied by sumfrequency generation. Surf. Sci. 360, 104–111 (1996).
Lazić, I. et al. Phase contrast STEM for thin samples: integrated differential phase contrast. Ultramicroscopy 160, 265–280 (2016).
Yücelen, E. et al. Phase contrast scanning transmission electron microscopy imaging of light and heavy atoms at the limit of contrast and resolution. Sci. Rep. 8, 2676 (2018).
Odoh, S. O. et al. Preferential location of germanium in the UTL and IPC-2a zeolites. J. Phys. Chem. C. 118, 26939–26946 (2014).
Kamakoti, P. et al. Role of germanium in the formation of double four rings in zeolites. J. Phys. Chem. C. 111, 3575–3583 (2007).
Sastre, G. et al. Preferential location of Ge atoms in polymorph C of beta zeolite (ITQ-17) and their structure-directing effect: a computational, XRD, and NMR spectroscopic study. Angew. Chem. Int. Ed. 41, 4722–4726 (2002).
Blasco, T. et al. Preferential Location of Ge in the double four-membered ring units of ITQ-7 zeolite. J. Phys. Chem. B 106, 2634–2642 (2002).
Liu, X. et al. Fluoride removal from double four-membered ring (D4R) units in as-synthesized Ge-containing zeolites. Chem. Mater. 23, 5052–5057 (2011).
Yang, M.-L. et al. First-principles calculations of propane dehydrogenation over PtSn catalysts. ACS Catal. 2, 1247–1258 (2012).
Zha, S. et al. Identification of Pt-based catalysts for propane dehydrogenation via a probability analysis. Chem. Sci. 9, 3925–3931 (2018).
Fricke, C. et al. Propane dehydrogenation on platinum catalysts: identifying the active sites through Bayesian analysis. ACS Catal. 12, 2487–2498 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 21872052, 21972044, 22172050 and 22105028), the National Key R&D Program of China (2021YFA1501401), ‘Grassland Talents’ of Inner Mongolia Autonomous Region, Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23030), ‘Steed plan High level Talents’ of Inner Mongolia University, and Fundamental Research Funds for the Central Universities (grant number 2022CDJXY-003). We gratefully acknowledge the BL17B beamline of the National Facility for Protein Science (NFPS), Shanghai Synchrotron Radiation Facility (SSRF) Shanghai, China for providing the beam time.
Author information
Authors and Affiliations
Contributions
P.W. conceived the project and directed the study. Y.M. carried out the synthesis, structural characterizations and catalytic measurements and wrote the manuscript. H.X. and Y.G. contributed to the experimental design and data interpretation. J.J. carried out the 19F MAS NMR measurements. L.Z. carried out the infrared CO-adsorption experiments. C.L., Y.Z. and X.W. assisted in the preparation method of the catalyst. L.L. and Y.H. carried out the Cs-corrected HAADF-STEM and iDPC-STEM measurements and images. J.Z. contributed to the collection and analysis of XAFS and PDF data in the BL17b beamline of the National Facility for Protein Science (NFPS), Shanghai Synchrotron Radiation Facility (SSRF). S.S. and W.S. carried out the theoretical calculations. All the authors discussed the results and contributed to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–3, Figs. 1–44, Tables 1–11 and refs. 1–8.
Supplementary Data
Atomic coordinates of the computational models.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, Y., Song, S., Liu, C. et al. Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation. Nat Catal 6, 506–518 (2023). https://doi.org/10.1038/s41929-023-00968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-00968-7
This article is cited by
-
Advances in in situ/operando techniques for catalysis research: enhancing insights and discoveries
Surface Science and Technology (2024)
-
The interaction of the structure-directing agent with the zeolite framework determines germanium distribution in SCM-15 germanosilicate
Frontiers of Chemical Science and Engineering (2024)
-
Trapped by the germanium ring
Nature Catalysis (2023)
-
CO2 emissions of constructing China’s power grid towards carbon–neutral target: Based on top-down and bottom-up integrated model
Environmental Science and Pollution Research (2023)