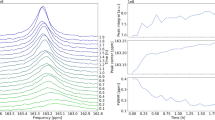
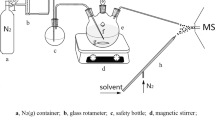
Abstract
Electrochemical carbon dioxide reduction is a potential pathway for sustainable production of fuels and chemicals. However, the detailed catalytic mechanism in cells using high-current gas diffusion electrodes remains uncertain. Here we use proton-transfer-reaction time-of-flight mass spectrometry (PTR–TOF–MS) to perform operando analysis of intermediates and products generated by electrochemical carbon dioxide reduction in gas-diffusion-electrode-based flow cells with copper-based electrocatalysts. PTR–TOF–MS allows for sensitive detection of C1–C4 minor and major intermediates and products, measurement of their 13C isotope composition and precise identification of onset potentials. We find that formaldehyde and acetaldehyde are not the major intermediates for formation of methanol and ethanol/ethylene, respectively, and that propionaldehyde reduction is on the major pathway for 1-propanol formation. Interestingly, the discrimination against 13C in the reaction products is substantially larger than for biological CO2 fixation in photosynthesis and Fischer–Tropsch synthesis of hydrocarbons.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data and analyses for the figures in the main part of the manuscript are openly available at https://doi.org/10.5281/zenodo.7047052. All data in the study are available from the corresponding authors upon reasonable request.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
De Arquer, F. P. G. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Liu, Z., Yang, H., Kutz, R. & Masel, R. I. CO2 electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371 (2018).
Kovalev, M. K., Ren, H., Zakir Muhamad, M., Ager, J. W. & Lapkin, A. A. Minor product polymerization causes failure of high-current CO2-to-ethylene electrolyzers. ACS Energy Lett. 7, 599–601 (2022).
Rangarajan, S., Maravelias, C. T. & Mavrikakis, M. Sequential-optimization-based framework for robust modeling and design of heterogeneous catalytic systems. J. Phys. Chem. C 121, 25847–25863 (2017).
Yang, Y., Mims, C. A., Mei, D., Peden, C. H. & Campbell, C. T. Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: the source of C in methanol and the role of water. J. Catal. 298, 10–17 (2013).
Pander, J. E. III, Ren, D. & Yeo, B. S. Practices for the collection and reporting of electrocatalytic performance and mechanistic information for the CO2 reduction reaction. Catal. Sci. Technol. 7, 5820–5832 (2017).
Grote, J.-P., Zeradjanin, A. R., Cherevko, S. & Mayrhofer, K. J. Coupling of a scanning flow cell with online electrochemical mass spectrometry for screening of reaction selectivity. Rev. Sci. Instrum. 85, 104101 (2014).
Schouten, K., Kwon, Y., Van Der Ham, C., Qin, Z. & Koper, M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2, 1902–1909 (2011).
Clark, E. L., Singh, M. R., Kwon, Y. & Bell, A. T. Differential electrochemical mass spectrometer cell design for online quantification of products produced during electrochemical reduction of CO2. Anal. Chem. 87, 8013–8020 (2015).
Schouten, K. J. P., Qin, Z., Pérez Gallent, E. & Koper, M. T. Two pathways for the formation of ethylene in CO reduction on single-crystal copper electrodes. J. Am. Chem. Soc. 134, 9864–9867 (2012).
Tsang, C. F. et al. Potential-dependent adsorption of CO and its low-overpotential reduction to CH3CH2OH on Cu (511) surface reconstructed from Cu (pc): operando studies by seriatim STM-EQCN-DEMS. J. Electrochem. Soc. 165, J3350 (2018).
Bondue, C. J. & Koper, M. T. A DEMS approach for the direct detection of CO formed during electrochemical CO2 reduction. J. Electroanal. Chem. 875, 113842 (2020).
Lobaccaro, P. et al. Initial application of selected-ion flow-tube mass spectrometry to real-time product detection in electrochemical CO2 reduction. Energy Technol. 6, 110–121 (2018).
Burdyny, T. & Smith, W. A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 12, 1442–1453 (2019).
Rabiee, H. et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review. Energy Environ. Sci. 14, 1959–2008 (2021).
Zardin, E., Tyapkova, O., Buettner, A. & Beauchamp, J. Performance assessment of proton-transfer-reaction time-of-flight mass spectrometry (PTR–TOF–MS) for analysis of isobaric compounds in food-flavour applications. Lebensm. Wiss. Technol. 56, 153–160 (2014).
Yuan, B. et al. Proton-transfer-reaction mass spectrometry: applications in atmospheric sciences. Chem. Rev. 117, 13187–13229 (2017).
Jordan, A. et al. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR–TOF–MS). Int. J. Mass spectrom. 286, 122–128 (2009).
O’Leary, M. H. Carbon isotopes in photosynthesis. Bioscience 38, 328–336 (1988).
Farquhar, G. D., Ehleringer, J. R. & Hubick, K. T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 40, 503–537 (1989).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Kohn, M. J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl Acad. Sci. USA 107, 19691–19695 (2010).
Taran, Y. A., Kliger, G. A. & Sevastianov, V. S. Carbon isotope effects in the open-system Fischer–Tropsch synthesis. Geochim. Cosmochim. Acta 71, 4474–4487 (2007).
Giri, S. D. & Sarkar, A. Electrochemical study of bulk and monolayer copper in alkaline solution. J. Electrochem. Soc. 163, H252–H259 (2016).
Choi, J. et al. Electrochemical CO2 reduction to CO on dendritic Ag–Cu electrocatalysts prepared by electrodeposition. Chem. Eng. Sci. 299, 37–44 (2016).
Lum, Y. & Ager, J. W. Stability of residual oxides in oxide-derived copper catalysts for electrochemical CO2 reduction investigated with 18O labeling. Angew. Chem. Int. Ed. 57, 551–554 (2018).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Wilson, P. F., Freeman, C. G. & McEwan, M. J. Reactions of small hydrocarbons with H3O+, O2+ and NO+ ions. Int. J. Mass Spectrom. 229, 143–149 (2003).
Myher, J. & Harrison, A. Some observations on the ion-molecule reactions in ethylene. Can. J. Chem. 46, 101–109 (1968).
Choi, M., Bong, S., Kim, J. W. & Lee, J. Formation of 1-butanol from CO2 without *CO dimerization on a phosphorus-rich copper cathode. ACS Energy Lett. 6, 2090–2095 (2021).
Hori, Y., Takahashi, R., Yoshinami, Y. & Murata, A. Electrochemical reduction of CO at a copper electrode. J. Phys. Chem. B 101, 7075–7081 (1997).
Boutin, E. et al. Aqueous electrochemical reduction of carbon dioxide and carbon monoxide into methanol with cobalt phthalocyanine. Angew. Chem. Int. Ed. 58, 16172–16176 (2019).
Cheng, T., Xiao, H. & Goddard, W. A. III Free-energy barriers and reaction mechanisms for the electrochemical reduction of CO on the Cu (100) surface, including multiple layers of explicit solvent at pH 0. J. Phys. Chem. Lett. 6, 4767–4773 (2015).
Nie, X., Esopi, M. R., Janik, M. J. & Asthagiri, A. Selectivity of CO2 reduction on copper electrodes: the role of the kinetics of elementary steps. Angew. Chem. Int. Ed. 125, 2519–2522 (2013).
Huang, Y., Chen, Y., Cheng, T., Wang, L.-W. & Goddard, W. A. III Identification of the selective sites for electrochemical reduction of CO to C2+ products on copper nanoparticles by combining reactive force fields, density functional theory, and machine learning. ACS Energy Lett. 3, 2983–2988 (2018).
Lum, Y. & Ager, J. W. Evidence for product-specific active sites on oxide-derived Cu catalysts for electrochemical CO2 reduction. Nat. Catal. 2, 86–93 (2019).
Luo, W., Nie, X., Janik, M. J. & Asthagiri, A. Facet dependence of CO2 reduction paths on Cu electrodes. ACS Catal. 6, 219–229 (2016).
Poulson, S. R. & Drever, J. I. Stable isotope (C, Cl, and H) fractionation during vaporization of trichloroethylene. Environ. Sci. Technol. 33, 3689–3694 (1999).
Bertheussen, E. et al. Acetaldehyde as an intermediate in the electroreduction of carbon monoxide to ethanol on oxide-derived copper. Angew. Chem. Int. Ed. 128, 1472–1476 (2016).
Zhang, X. & Zhou, Z. Perspective on theoretical models for CO2 electrochemical reduction. J. Phys. Chem. C 126, 3820–3829 (2022).
Ren, D., Wong, N. T., Handoko, A. D., Huang, Y. & Yeo, B. S. Mechanistic insights into the enhanced activity and stability of agglomerated Cu nanocrystals for the electrochemical reduction of carbon dioxide to n-propanol. J. Phys. Chem. Lett. 7, 20–24 (2016).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 1–9 (2018).
Pătru, A., Binninger, T., Pribyl, B. & Schmidt, T. J. Design principles of bipolar electrochemical co-electrolysis cells for efficient reduction of carbon dioxide from gas phase at low temperature. J. Electrochem. Soc. 166, F34 (2019).
Goericke, R. & Fry, B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochem. Cycles 8, 85–90 (1994).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 1–7 (2015).
Salazar Gómez, J. I. et al. Elucidation of artefacts in proton transfer reaction time-of-flight mass spectrometers. J. Mass Spectrom. 54, 987–1002 (2019).
Fleisher, A. J. et al. Absolute 13C/12C isotope amount ratio for Vienna PeeDee Belemnite from infrared absorption spectroscopy. Nat. Phys. 17, 889–893 (2021).
Millard, P. et al. IsoCor: isotope correction for high-resolution MS labeling experiments. Bioinformatics 35, 4484–4487 (2019).
Su, X., Lu, W. & Rabinowitz, J. D. Metabolite spectral accuracy on orbitraps. Anal. Chem. 89, 5940–5948 (2017).
Tcherkez, G., Mahé, A. & Hodges, M. 12C/13C fractionations in plant primary metabolism. Trends Plant Sci. 16, 499–506 (2011).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Methfessel, M. & Paxton, A. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 40, 3616 (1989).
Nørskov, J. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Acknowledgements
This work was supported by the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme through the eCO2EP project operated by Cambridge Centre for Advanced Research and Education in Singapore (CARES) and the Berkeley Education Alliance for Research in Singapore (BEARS). We thank E. Hartungen of IONICON for valuable technical advice on the operation of the PTR-MS instrument.
Author information
Authors and Affiliations
Contributions
H.R. conceptualized the study, developed the methodology and software, performed experiments, analysed and validated data, wrote the original draft of manuscript and revised the manuscript. M.K. developed the methodology, performed experiments, analysed and validated data and reviewed the manuscript. Z.W. performed DFT calculations with assistance from H.M. and wrote the DFT parts of the manuscript. M.Z.M., Y.S., L.S., J.W. and W.Y. assisted in experiments. S.R. contributed to mechanism analysis. A.A.L. acquired funding, supervised the project and reviewed the manuscript. J.W.A. conceptualized the study, acquired funding, developed the methodology and software, administered the project, supervised the project and reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Leanne Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–5, Figs. 1–28 and Tables 1–10.
Supplementary Data 1
Atomic coordinates of intermediates and products used in DFT calculations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, H., Kovalev, M., Weng, Z. et al. Operando proton-transfer-reaction time-of-flight mass spectrometry of carbon dioxide reduction electrocatalysis. Nat Catal 5, 1169–1179 (2022). https://doi.org/10.1038/s41929-022-00891-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00891-3
This article is cited by
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Direct time-resolved observation of surface-bound carbon dioxide radical anions on metallic nanocatalysts
Nature Communications (2023)
-
Mass spec live
Nature Catalysis (2022)