Abstract
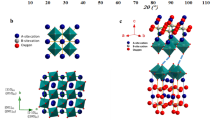
The biggest obstacle to the commercialization of protonic ceramic fuel cells (PCFCs) is the lack of high-performance, low-cost cathode materials. Currently, the most promising cathode materials are cobalt-based perovskites; however, the unstable phases, poor thermomechanical compatibility with other PCFC components, high cost and unsatisfactory performance limit the viability of these materials. Here we combine ab initio simulations, molecular orbital insights, and A- and B-site co-substitution to develop a cobalt-free perovskite with outstanding performance. A- and B-site substitution in BaFeO3−δ, is found to promote the formation of oxygen vacancies (\({{{\mathrm{V}}}}_{{{\mathrm{O}}}}^{ \bullet \bullet }\)) and hydroxyl ions (\({{{\mathrm{OH}}}}_{{{\mathrm{O}}}}^ \bullet\)) while retaining structural stability. The best computationally identified material, Ba0.875Fe0.875Zr0.125O3−δ, showed exceptional oxygen reduction reaction electrochemical activity with a peak power density of 0.67 W cm−2 at 500 °C. This rational approach provides a strategy for designing high-activity, low-cost and cobalt-free perovskites, marking a significant step towards realizing commercially viable PCFCs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files or from the corresponding author upon reasonable request. The atomic coordinates of the optimized structures are provided in Supplementary Data 1. Source data are provided with this paper.
References
Adler, S. B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 104, 4791–4844 (2004).
Ormerod, R. M. Solid oxide fuel cells. Chem. Soc. Rev. 32, 17–28 (2003).
Brett, D. J. L., Atkinson, A., Brandon, N. P. & Skinner, S. J. Intermediate temperature solid oxide fuel cells. Chem. Soc. Rev. 37, 1568–1578 (2008).
Krishnan, V. V. Recent developments in metal-supported solid oxide fuel cells. WIREs Energy Environ. 6, e246 (2017).
Staffell, I. et al. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463–491 (2019).
Gao, Z., Mogni, L. V., Miller, E. C., Railsback, J. G. & Barnett, S. A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 9, 1602–1644 (2016).
Duan, C., Huang, J., Sullivan, N. & O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 7, 011314 (2020).
Zohourian, R., Merkle, R., Raimondi, G. & Maier, J. Mixed-conducting perovskites as cathode materials for protonic ceramic fuel cells: understanding the trends in proton uptake. Adv. Funct. Mater. 28, 1801241 (2018).
Zhu, H. & Kee, R. J. Modeling protonic-ceramic fuel cells with porous composite electrodes in a button-cell configuration. J. Electrochem. Soc. 164, F1400–F1411 (2017).
Matsuzaki, Y. et al. Effect of proton-conduction in electrolyte on electric efficiency of multi-stage solid oxide fuel cells. Sci. Rep. 5, 12640 (2015).
Duan, C. et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 349, 1321–1326 (2015).
Duan, C. et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 557, 217–222 (2018).
Choi, S. et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3, 202–210 (2018).
An, H. et al. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm–2 at 600 °C. Nat. Energy 3, 870–875 (2018).
Shim, J. H. Ceramics breakthrough. Nat. Energy 3, 168–169 (2018).
Zhang, Y. et al. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 29, 1700132 (2017).
Kreuer, K. D. et al. Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ion. 145, 295–306 (2001).
Duan, C., Hook, D., Chen, Y., Tong, J. & O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 10, 176–182 (2017).
Li, M. et al. Smart utilization of cobaltite-based double perovskite cathodes on barrier-layer-free zirconia electrolyte of solid oxide fuel cells. J. Mater. Chem. A 4, 19019–19025 (2016).
Song, Y. et al. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 3, 2842–2853 (2019).
Wang, Z. et al. A high performance cathode for proton conducting solid oxide fuel cells. J. Mater. Chem. A 3, 8405–8412 (2015).
Poetzsch, D., Merkle, R. & Maier, J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys. Chem. Chem. Phys. 16, 16446–16453 (2014).
Fabbri, E., Markus, I., Bi, L., Pergolesi, D. & Traversa, E. Tailoring mixed proton-electronic conductivity of BaZrO3 by Y and Pr co-doping for cathode application in protonic SOFCs. Solid State Ion. 202, 30–35 (2011).
Tao, Z., Bi, L., Zhu, Z. & Liu, W. Novel cobalt-free cathode materials BaCexFe1−xO3−δ for proton-conducting solid oxide fuel cells. J. Power Sources 194, 801–804 (2009).
Xia, Y. et al. A novel BaFe0.8Zn0.1Bi0.1O3−δ cathode for proton conducting solid oxide fuel cells. Ceram. Int. 46, 25453–25459 (2020).
Xu, X. et al. Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J. Mater. Chem. A 7, 18792–18798 (2019).
Wang, N. et al. Mixed proton–electron–oxide ion triple conducting manganite as an efficient cobalt-free cathode for protonic ceramic fuel cells. J. Mater. Chem. A 8, 11043–11055 (2020).
Gu, C.-Y. et al. High performance Ca-containing La2−xCaxNiO4+δ (0 ≤ x ≤ 0.75) cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrog. Energy 45, 23422–23432 (2020).
Wang, Z. et al. Ba0.95La0.05Fe0.8Zn0.2O3−δ cobalt-free perovskite as a triple-conducting cathode for proton-conducting solid oxide fuel cells. Ceram. Int. 46, 18216–18223 (2020).
Xia, Y. et al. A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J. Mater. Chem. A 7, 16136–16148 (2019).
Knöchel, P. L. et al. Synthesis, structural characterisation and proton conduction of two new hydrated phases of barium ferrite BaFeO2.5−x(OH)2x. J. Mater. Chem. A 4, 3415–3430 (2016).
Cheng, S. et al. A dual-phase ceramic membrane with extremely high H2 permeation flux prepared by autoseparation of a ceramic precursor. Angew. Chem. Int. Ed. 55, 10895–10898 (2016).
Papac, M., Stevanović, V., Zakutayev, A. & O’Hayre, R. Triple ionic–electronic conducting oxides for next-generation electrochemical devices. Nat. Mater. 20, 301–313 (2020).
Choi, S., Davenport, T. C. & Haile, S. M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 12, 206–215 (2019).
Pei, K. et al. Surface restructuring of a perovskite-type air electrode for reversible protonic ceramic electrochemical cells. Nat. Commun. 13, 2207 (2022).
Zhang, H. et al. An efficient and durable anode for ammonia protonic ceramic fuel cells. Energy Environ. Sci. 15, 287–295 (2022).
Hayashi, N. et al. BaFeO3: a ferromagnetic iron oxide. Angew. Chem. Int. Ed. 50, 12547–12550 (2011).
Hoedl, M. F., Gryaznov, D., Merkle, R., Kotomin, E. A. & Maier, J. Interdependence of oxygenation and hydration in mixed conducting (Ba,Sr)FeO3−δ perovskites studied by density functional theory. J. Phys. Chem. C 124, 11780–11289 (2020).
Muñoz-García, A. B. & Pavone, M. First-principles design of new electrodes for proton-conducting solid-oxide electrochemical cells: A-site doped Sr2Fe1.5Mo0.5O6−δ perovskite. Chem. Mater. 28, 490–500 (2016).
Muñoz-García, A. B., Tuccillo, M. & Pavone, M. Computational design of cobalt-free mixed proton–electron conductors for solid oxide electrochemical cells. J. Mater. Chem. A 5, 11825–11833 (2017).
Zhang, Z. et al. New horizons for inorganic solid state ion conductors. Energy Environ. Sci. 11, 1945–1976 (2018).
Madsen, G. K. H. & Singh, D. J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 175, 67–71 (2006).
Madsen, G. K. H., Carrete, J. & Verstraete, M. J. BoltzTraP2, a program for interpolating band structures and calculating semi-classical transport coefficients. Comput. Phys. Commun. 231, 140–145 (2018).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: a user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Dong, F., Chen, D., Chen, Y., Zhao, Q. & Shao, Z. La-doped BaFeO3−δ perovskite as a cobalt-free oxygen reduction electrode for solid oxide fuel cells with oxygen-ion conducting electrolyte. J. Mater. Chem. 22, 15071–15079 (2012).
Wang, J. et al. The effect of A-site and B-site substitution on BaFeO3−δ: an investigation as a cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 297, 511–518 (2015).
Chen, C. & Ciucci, F. Designing Fe-based oxygen catalysts by density functional theory calculations. Chem. Mater. 28, 7058–7065 (2016).
Baiyee, Z. M., Chen, C. & Ciucci, F. A DFT+U study of A-site and B-site substitution in BaFeO3−δ. Phys. Chem. Chem. Phys. 17, 23511–23520 (2015).
Pearson, R. G. Hard and soft acids and bases, HSAB, part 1: fundamental principles. J. Chem. Educ. 45, 581 (1968).
Pearson, R. G. Hard and soft acids and bases, HSAB, part II: underlying theories. J. Chem. Educ. 45, 643 (1968).
Liu, X. et al. Lattice characteristics, structure stability and oxygen permeability of BaFe1−xYxO3−δ ceramic membranes. J. Membr. Sci. 383, 235–240 (2011).
Cao, Y., Gadre, M. J., Ngo, A. T., Adler, S. B. & Morgan, D. D. Factors controlling surface oxygen exchange in oxides. Nat. Commun. 10, 1346 (2019).
Huang, Y.-L., Pellegrinelli, C. & Wachsman, E. D. Oxygen dissociation kinetics of concurrent heterogeneous reactions on metal oxides. ACS Catal. 7, 5766–5772 (2017).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Jung, W. & Tuller, H. L. A new model describing solid oxide fuel cell cathode kinetics: model thin film SrTi1−xFexO3−δ mixed conducting oxides—a case study. Adv. Energy Mater. 1, 1184–1191 (2011).
Raimondi, G. et al. X-ray spectroscopy of (Ba,Sr,La)(Fe,Zn,Y)O3−δ identifies structural and electronic features favoring proton uptake. Chem. Mater. 32, 8502–8511 (2020).
Vračar, M. et al. Jahn-Teller distortion around Fe4+ in Sr(FexTi1−x)O3−δ from X-ray absorption spectroscopy, X-ray diffraction, and vibrational spectroscopy. Phys. Rev. B 76, 174107 (2007).
Zhou, C. et al. Realizing stable high hydrogen permeation flux through BaCo0.4Fe0.4Zr0.1Y0.1O3−δ membrane using a thin Pd film protection strategy. J. Membr. Sci. 596, 117709 (2020).
Song, S. J., Wachsman, E. D., Rhodes, J., Dorris, S. E. & Balachandran, U. Hydrogen permeability of SrCe1−xMxO3−δ (x = 0.05, M = Eu, Sm). Solid State Ion. 167, 99–105 (2004).
Chen, Y., Liu, H., Zhuang, L., Wei, Y. & Wang, H. Hydrogen permeability through Nd5.5W0.35Mo0.5Nb0.15O11.25−δ mixed protonic–electronic conducting membrane. J. Membr. Sci. 579, 33–39 (2019).
Yang, Y., Zeng, Y., Amirkhiz, B. S., Luo, J.-L. & Yan, N. Promoting the ambient-condition stability of Zr-doped barium cerate: toward robust solid oxide fuel cells and hydrogen separation in syngas. J. Power Sources 378, 134–138 (2018).
Zuo, C., Dorris, S. E., Balachandran, U. & Liu, M. Effect of Zr-doping on the chemical stability and hydrogen permeation of the Ni–BaCe0.8Y0.2O3−α mixed protonic–electronic conductor. Chem. Mater. 18, 4647–4650 (2006).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Zhang, W. et al. Insights into ionic transport and structural changes in magnetite during multiple-electron transfer reactions. Adv. Energy Mater. 6, 1502471 (2016).
Yang, L. et al. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2–xYbxO3–δ. Science 326, 126–129 (2009).
Acknowledgements
The authors gratefully acknowledge the Research Grant Council of Hong Kong for support through the projects (16201820 and 16206019, received by F.C.), the Nano & Material Technology Development Project (NRF-2021M3H4A1A01002919, received by F.C.) and the Global PhD Fellowship through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science, ICT, and Future Planning (NRF-2018H1A2A1060644, received by A.S.). This work was supported in part by the Project of Hetao Shenzhen-Hong Kong Science and Technology Innovation Cooperation Zone (HZQB-KCZYB-2020083, received by F.C.). M.J.R. and A.B. kindly recognize the support of the Hong Kong PhD Fellowship Scheme. We are grateful to the Materials Characterization and Preparation Facility (MCPF) and the Advanced Engineering Materials Facility (AEMF) of the Hong Kong University of Science and Technology for their assistance in experimental characterizations. The calculations were performed on the Tianhe-2 supercomputer system in Guangzhou.
Author information
Authors and Affiliations
Contributions
Z.W., Y.W. and F.C. conceived and designed the project. Z.W. performed theoretical calculations. Y.W. fabricated fuel cells and performed X-ray diffraction and SEM analyses. Y.S., M.Y., A.S., G.K. and Z.S. measured proton conductivity. J.W. and J.Lim performed X-ray absorption spectroscopy characterizations and contributed to data analysis. Z.Z., A.B. and J.Liu performed TEM characterizations. Z.W., Y.W., Y.S., M.J.R. and F.C. drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Chuancheng Duan, Kevin Huang and Ivano Castelli for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–42, Tables 1–12, methods and references.
Supplementary Data
Contains the coordinates of the calculated species in this article.
Source data
Source Data Fig. 1
Contains the energies of calculated species.
Source Data Fig. 2
Contains the energies of calculated species.
Source Data Fig. 3
The projected density of state of Fe–O, Zr–O and Y–O bonds
Source Data Fig. 5
Contains the energies of calculated intermediates of oxygen reduction reactions for BFZ, BFZ-1Vo, BFZ-2Vo and BFZ-2Vo-2OHo
Source Data Fig. 6
The data used to plot the figures.
Source Data Fig. 7
The data used to plot the figures.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Wang, Y., Wang, J. et al. Rational design of perovskite ferrites as high-performance proton-conducting fuel cell cathodes. Nat Catal 5, 777–787 (2022). https://doi.org/10.1038/s41929-022-00829-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00829-9
This article is cited by
-
Recent progress in oxygen electrodes for protonic ceramic electrochemical cells
Journal of the Korean Ceramic Society (2024)
-
Rational Design of Ruddlesden–Popper Perovskite Ferrites as Air Electrode for Highly Active and Durable Reversible Protonic Ceramic Cells
Nano-Micro Letters (2024)
-
Sintering-induced cation displacement in protonic ceramics and way for its suppression
Nature Communications (2023)
-
Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C
Nature Energy (2023)
-
Simulations guide the optimization of fuel cell cathodes
Nature Reviews Materials (2022)