Abstract
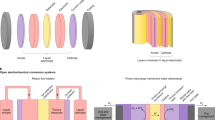
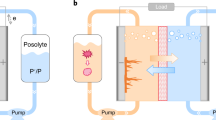
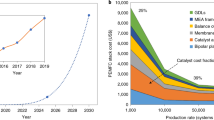
Cost-competitive fuel cells and water electrolysers require highly efficient electrocatalysts for the respective reactions of hydrogen oxidation and evolution, and oxygen evolution and reduction. Electrocatalyst activity and durability are commonly assessed using rotating disk electrodes (RDEs) or membrane electrode assemblies (MEAs). RDEs provide a quick and widely accessible testing tool, whereas MEA testing is more complex but closely resembles the actual application. Although both experimental set-ups allow investigation of the same reactions, there are scientific questions that cannot be answered by the RDE technique. In this Perspective, we scrutinize protocols widely used to determine the activity and durability of electrocatalysts, and highlight discrepancies in the results obtained using RDEs and MEAs. We discuss where the use of RDEs is appropriate and, conversely, where it leads to erroneous interpretations. Ultimately, we show that many of the current challenges for hydrogen and oxygen electrocatalysts require MEA testing and advocate for its greater adoption in the early stages of electrocatalyst development.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Buttler, A. & Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew. Sustain. Energy Rev. 82, 2440–2454 (2018).
Fan, J. et al. Bridging the gap between highly active oxygen reduction reaction catalysts and effective catalyst layers for proton exchange membrane fuel cells. Nat. Energy 6, 475–486 (2021).
Kodama, K., Nagai, T., Kuwaki, A., Jinnouchi, R. & Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 16, 140–147 (2021).
Shinozaki, K., Zack, J. W., Richards, R. M., Pivovar, B. S. & Kocha, S. S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique: I. Impact of impurities, measurement protocols and applied corrections. J. Electrochem. Soc. 162, F1144–F1158 (2015).
Gasteiger, H. A., Panels, J. E. & Yan, S. G. Dependence of PEM fuel cell performance on catalyst loading. J. Power Sources 127, 162–171 (2004).
Schmidt, T. J. et al. Characterization of high‐surface‐area electrocatalysts using a rotating disk electrode configuration. J. Electrochem. Soc. 145, 2354–2358 (1998).
Wakabayashi, N., Takeichi, M., Itagaki, M., Uchida, H. & Watanabe, M. Temperature-dependence of oxygen reduction activity at a platinum electrode in an acidic electrolyte solution investigated with a channel flow double electrode. J. Electroanal. Chem. 574, 339–346 (2005).
Schröder, J. et al. The gas diffusion electrode setup as straightforward testing device for proton exchange membrane water electrolyzer catalysts. JACS Au 1, 247–251 (2021).
Ehelebe, K. et al. Evaluating electrocatalysts at relevant currents in a half-cell: the impact of Pt loading on oxygen reduction reaction. J. Electrochem. Soc. 166, F1259–F1268 (2019).
Gasteiger, H. A., Kocha, S. S., Sompalli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 56, 9–35 (2005).
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications Vol. 2 (Wiley, 2001).
Garsany, Y., Ge, J., St-Pierre, J., Rocheleau, R. & Swider-Lyons, K. E. Analytical procedure for accurate comparison of rotating disk electrode results for the oxygen reduction activity of Pt/C. J. Electrochem. Soc. 161, F628–F640 (2014).
Kocha, S. S. et al. Best practices and testing protocols for benchmarking ORR activities of fuel cell electrocatalysts using rotating disk electrode. Electrocatalysis 8, 366–374 (2017).
Fathi Tovini, M., Hartig-Weiß, A., Gasteiger, H. A. & El-Sayed, H. A. The discrepancy in oxygen evolution reaction catalyst lifetime explained: RDE vs MEA - dynamicity within the catalyst layer matters. J. Electrochem. Soc. 168, 014512 (2021).
Pan, L., Ott, S., Dionigi, F. & Strasser, P. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 18, 61–71 (2019).
Jiang, J. & Kucernak, A. Investigations of fuel cell reactions at the composite microelectrode|solid polymer electrolyte interface. I. Hydrogen oxidation at the nanostructured Pt|Nafion® membrane interface. J. Electroanal. Chem. 567, 123–137 (2004).
Chen, S. & Kucernak, A. Electrocatalysis under conditions of high mass transport rate: oxygen reduction on single submicrometer-sized Pt particles supported on carbon. J. Phys. Chem. B 108, 3262–3276 (2004).
Zalitis, C. M., Kramer, D. & Kucernak, A. R. Electrocatalytic performance of fuel cell reactions at low catalyst loading and high mass transport. Phys. Chem. Chem. Phys. 15, 4329–4340 (2013).
Petzoldt, P. J., Kwan, J. T. H., Bonakdarpour, A. & Wilkinson, D. P. Deconvoluting reversible and irreversible degradation phenomena in OER catalyst coated membranes using a modified RDE technique. J. Electrochem. Soc. 168, 026507 (2021).
Martens, S. et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 392, 274–284 (2018).
Katsounaros, I. et al. The effective surface pH during reactions at the solid–liquid interface. Electrochem. Commun. 13, 634–637 (2011).
Okada, T., Møller-Holst, S., Gorseth, O. & Kjelstrup, S. Transport and equilibrium properties of Nafion® membranes with H+ and Na+ ions. J. Electroanal. Chem. 442, 137–145 (1998).
Ito, H., Maeda, T., Nakano, A. & Takenaka, H. Properties of Nafion membranes under PEM water electrolysis conditions. Int. J. Hydrogen Energy 36, 10527–10540 (2011).
Ehelebe, K., Escalera-López, D. & Cherevko, S. Limitations of aqueous model systems in the stability assessment of electrocatalysts for oxygen reactions in fuel cell and electrolyzers. Curr. Opin. Electrochem. 29, 100832 (2021).
Cheng, X. et al. A review of PEM hydrogen fuel cell contamination: impacts, mechanisms, and mitigation. J. Power Sources 165, 739–756 (2007).
Greszler, T. A., Moylan, T. E. & Gasteiger, H. A. in Handbook of Fuel Cells: Fundamentals, Technology and Applications Vol. 6: Advances in Electrocatalysis, Materials, Diagnostics and Durability (eds Vielstich, W. et al.) 729–748 (Wiley, 2009).
Papadias, D. D. et al. Durability of Pt–Co alloy polymer electrolyte fuel cell cathode catalysts under accelerated stress tests. J. Electrochem. Soc. 165, F3166–F3177 (2018).
Ahluwalia, R. K. et al. Durability of de-alloyed platinum-nickel cathode catalyst in low platinum loading membrane-electrode assemblies subjected to accelerated stress tests. J. Electrochem. Soc. 165, F3316–F3327 (2018).
Durst, J., Chatenet, M. & Maillard, F. Impact of metal cations on the electrocatalytic properties of Pt/C nanoparticles at multiple phase interfaces. Phys. Chem. Chem. Phys. 14, 13000–13009 (2012).
Jovanovič, P. et al. New insight into platinum dissolution from nanoparticulate platinum-based electrocatalysts using highly sensitive in situ concentration measurements. ChemCatChem 6, 449–453 (2014).
Ayers, K. et al. Perspectives on low-temperature electrolysis and potential for renewable hydrogen at scale. Annu. Rev. Chem. Biomol. Eng. 10, 219–239 (2019).
Ohno, H. et al. Remarkable mass activities for the oxygen evolution reaction at iridium oxide nanocatalysts dispersed on tin oxides for polymer electrolyte membrane water electrolysis. J. Electrochem. Soc. 164, F944–F947 (2017).
Hartig-Weiss, A. et al. Iridium oxide catalyst supported on antimony-doped tin oxide for high oxygen evolution reaction activity in acidic media. ACS Appl. Nano Mater. 3, 2185–2196 (2020).
Alia, S. M. & Anderson, G. C. Iridium oxygen evolution activity and durability baselines in rotating disk electrode half-cells. J. Electrochem. Soc. 166, F282–F294 (2019).
Bernt, M. et al. Current challenges in catalyst development for PEM water electrolyzers. Chem. Ing. Tech. 92, 31–39 (2020).
Oakton, E. et al. IrO2–TiO2: a high-surface-area, active, and stable electrocatalyst for the oxygen evolution reaction. ACS Catal. 7, 2346–2352 (2017).
Bernt, M. et al. Effect of the IrOx conductivity on the anode electrode/porous transport layer interfacial resistance in PEM water electrolyzers. J. Electrochem. Soc. 168, 084513 (2021).
Geiger, S. et al. Catalyst stability benchmarking for the oxygen evolution reaction: the importance of backing electrode material and dissolution in accelerated aging studies. ChemSusChem 10, 4140–4143 (2017).
Oh, H.-S. et al. Electrochemical catalyst–support effects and their stabilizing role for IrOx nanoparticle catalysts during the oxygen evolution reaction. J. Am. Chem. Soc. 138, 12552–12563 (2016).
El-Sayed, H. A., Weiß, A., Olbrich, L. F., Putro, G. P. & Gasteiger, H. A. OER catalyst stability investigation using RDE technique: a stability measure or an artifact? J. Electrochem. Soc. 166, F458–F464 (2019).
Knöppel, J. et al. On the limitations in assessing stability of oxygen evolution catalysts using aqueous model electrochemical cells. Nat. Commun. 12, 2231 (2021).
Hartig-Weiss, A., Tovini, M. F., Gasteiger, H. A. & El-Sayed, H. A. OER catalyst durability tests using the rotating disk electrode technique: the reason why this leads to erroneous conclusions. ACS Appl. Energy Mater. 3, 10323–10327 (2020).
Tan, X., Shen, J., Semagina, N. & Secanell, M. Decoupling structure-sensitive deactivation mechanisms of Ir/IrOx electrocatalysts toward oxygen evolution reaction. J. Catal. 371, 57–70 (2019).
Zalitis, C. M., Sharman, J., Wright, E. & Kucernak, A. R. Properties of the hydrogen oxidation reaction on Pt/C catalysts at optimised high mass transport conditions and its relevance to the anode reaction in PEFCs and cathode reactions in electrolysers. Electrochim. Acta 176, 763–776 (2015).
Durst, J., Simon, C., Hasché, F. & Gasteiger, H. A. Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J. Electrochem. Soc. 162, F190–F203 (2015).
Bernt, M., Siebel, A. & Gasteiger, H. A. Analysis of voltage losses in PEM water electrolyzers with low platinum group metal loadings. J. Electrochem. Soc. 165, F305–F314 (2018).
Sheng, W., Gasteiger, H. A. & Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: acid vs alkaline electrolytes. J. Electrochem. Soc. 157, B1529 (2010).
Genorio, B. et al. Selective catalysts for the hydrogen oxidation and oxygen reduction reactions by patterning of platinum with calix[4]arene molecules. Nat. Mater. 9, 998–1003 (2010).
Zheng, J., Yan, Y. & Xu, B. Correcting the hydrogen diffusion limitation in rotating disk electrode measurements of hydrogen evolution reaction kinetics. J. Electrochem. Soc. 162, F1470–F1481 (2015).
Chen, Q. & Luo, L. Correlation between gas bubble formation and hydrogen evolution reaction kinetics at nanoelectrodes. Langmuir 34, 4554–4559 (2018).
Saibi, R., Punathil Meethal, R. & Srinivasan, R. Mechanistic analysis of hydrogen evolution reaction on Pt in HClO4 using inverted rotating disc electrode. Electroanalysis 32, 2545–2554 (2020).
Elbert, K. et al. Elucidating hydrogen oxidation/evolution kinetics in base and acid by enhanced activities at the optimized Pt shell thickness on the Ru core. ACS Catal. 5, 6764–6772 (2015).
Rheinländer, P. J., Herranz, J., Durst, J. & Gasteiger, H. A. Kinetics of the hydrogen oxidation/evolution reaction on polycrystalline platinum in alkaline electrolyte reaction order with respect to hydrogen pressure. J. Electrochem. Soc. 161, F1448–F1457 (2014).
Zheng, J., Sheng, W., Zhuang, Z., Xu, B. & Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2, e1501602 (2016).
Stühmeier, B. M., Pietsch, M. R., Schwämmlein, J. N. & Gasteiger, H. A. Pressure and temperature dependence of the hydrogen oxidation and evolution reaction kinetics on Pt electrocatalysts via PEMFC-based hydrogen-pump measurements. J. Electrochem. Soc. 168, 064516 (2021).
Fuel Cell Technologies Program Multi-Year Research, Development, and Demonstration Plan—3.4 Fuel Cells (US Department of Energy, 2017); https://www.energy.gov/eere/fuelcells/downloads/hydrogen-and-fuel-cell-technologies-office-multi-year-research-development
Asset, T. et al. A review on recent developments and prospects for the oxygen reduction reaction on hollow Pt-alloy nanoparticles. ChemPhysChem 19, 1552–1567 (2018).
Zaman, S. et al. Oxygen reduction electrocatalysts toward practical fuel cells: progress and perspectives. Angew. Chem. Int. Ed. 60, 2–23 (2021).
Ly, A., Asset, T. & Atanassov, P. Integrating nanostructured Pt-based electrocatalysts in proton exchange membrane fuel cells. J. Power Sources 478, 228516 (2020).
Inaba, M. et al. Benchmarking high surface area electrocatalysts in a gas diffusion electrode: measurement of oxygen reduction activities under realistic conditions. Energy Environ. Sci. 11, 988–994 (2018).
Mayrhofer, K. J. J. et al. Fuel cell catalyst degradation on the nanoscale. Electrochem. Commun. 10, 1144–1147 (2008).
Meier, J. C. et al. Stability investigations of electrocatalysts on the nanoscale. Energy Environ. Sci. 5, 9319–9330 (2012).
Hu, Y. et al. Revealing the genuine stability of the reference Pt/C electrocatalyst toward the ORR. Electrochim. Acta 391, 138963 (2021).
Stariha, S. et al. Recent advances in catalyst accelerated stress tests for polymer electrolyte membrane fuel cells. J. Electrochem. Soc. 165, F492–F501 (2018).
Riese, A., Banham, D., Ye, S. & Sun, X. Accelerated stress testing by rotating disk electrode for carbon corrosion in fuel cell catalyst supports. J. Electrochem. Soc. 162, F783–F788 (2015).
Reiser, C. A. et al. A reverse-current decay mechanism for fuel cells. Electrochem. Solid State Lett. 8, A273–A276 (2005).
Nagai, T., Murata, H. & Morimoto, Y. Influence of experimental conditions on the catalyst degradation in the durability test. J. Electrochem. Soc. 161, F789–F794 (2014).
Schröder, J. et al. A new approach to probe the degradation of fuel cell catalysts under realistic conditions: combining tests in a gas diffusion electrode setup with small angle X-ray scattering. J. Electrochem. Soc. 167, 134515 (2020).
Kneer, A. & Wagner, N. A semi-empirical catalyst degradation model based on voltage cycling under automotive operating conditions in PEM fuel cells. J. Electrochem. Soc. 166, F120–F127 (2019).
Kongkanand, A. & Mathias, M. F. The priority and challenge of high-power performance of low-platinum proton-exchange membrane fuel cells. J. Phys. Chem. Lett. 7, 1127–1137 (2016).
Gittleman, C. S., Kongkanand, A., Masten, D. & Gu, W. Materials research and development focus areas for low cost automotive proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 18, 81–89 (2019).
Baker, D. R., Caulk, D. A., Neyerlin, K. C. & Murphy, M. W. Measurement of oxygen transport resistance in PEM fuel cells by limiting current methods. J. Electrochem. Soc. 156, B991 (2009).
Liu, Y. et al. Proton conduction and oxygen reduction kinetics in PEM fuel cell cathodes: effects of ionomer-to-carbon ratio and relative humidity. J. Electrochem. Soc. 156, B970–B980 (2009).
Harzer, G. S., Orfanidi, A., El-Sayed, H., Madkikar, P. & Gasteiger, H. A. Tailoring catalyst morphology towards high performance for low Pt loaded PEMFC cathodes. J. Electrochem. Soc. 165, F770–F779 (2018).
Owejan, J. P., Owejan, J. E. & Gu, W. Impact of platinum loading and catalyst layer structure on PEMFC performance. J. Electrochem. Soc. 160, F824–F833 (2013).
Park, Y.-C., Tokiwa, H., Kakinuma, K., Watanabe, M. & Uchida, M. Effects of carbon supports on Pt distribution, ionomer coverage and cathode performance for polymer electrolyte fuel cells. J. Power Sources 315, 179–191 (2016).
Yarlagadda, V. et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 3, 618–621 (2018).
Lazaridis, T. & Gasteiger, H. A. Pt-catalyzed oxidation of PEMFC carbon supports: a path to highly accessible carbon morphologies and implications for start-up/shut-down degradation. J. Electrochem. Soc. 168, 114517 (2021).
Ramaswamy, N. et al. Editors’ choice—ionomer side chain length and equivalent weight impact on high current density transport resistances in PEMFC cathodes. J. Electrochem. Soc. 168, 024518 (2021).
Orfanidi, A. et al. The key to high performance low Pt loaded electrodes. J. Electrochem. Soc. 164, F418–F426 (2017).
Spöri, C., Kwan, J. T. H., Bonakdarpour, A., Wilkinson, D. P. & Strasser, P. The stability challenges of oxygen evolving catalysts: towards a common fundamental understanding and mitigation of catalyst degradation. Angew. Chem. Int. Ed. 56, 5994–6021 (2017).
Ramaswamy, N., Gu, W., Ziegelbauer, J. M. & Kumaraguru, S. Carbon support microstructure impact on high current density transport resistances in PEMFC cathode. J. Electrochem. Soc. 167, 064515 (2020).
Acknowledgements
T.L. acknowledges funding from the Swiss National Foundation under the funding scheme Sinergia (project grant number 180335). B.M.S. acknowledges support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2089/1–390776260). We thank our colleague P. Rapp for the graphic design of Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the overall structure of this Perspective. T.L., B.M.S. and H.A.E.-S. co-wrote the manuscript, with T.L. and B.M.S. contributing the discussion of general aspects and the conclusion, H.A.E.-S. focusing on the OER section, B.M.S. drafting the HOR/HER section and T.L. discussing the ORR-specific aspects. All authors provided insights, contributed feedback and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Jiujun Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lazaridis, T., Stühmeier, B.M., Gasteiger, H.A. et al. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat Catal 5, 363–373 (2022). https://doi.org/10.1038/s41929-022-00776-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-022-00776-5
This article is cited by
-
And yet it rotates!
Nature Catalysis (2024)
-
Correlative Mn-Co catalyst excels Pt in oxygen reduction reaction of quasi-solid-state zinc-air batteries
Nano Research (2024)
-
Blocking the sulfonate group in Nafion to unlock platinum’s activity in membrane electrode assemblies
Nature Catalysis (2023)
-
Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers
Nature Communications (2023)
-
High-performance ionomerless cathode anion-exchange membrane fuel cells with ultra-low-loading Ag–Pd alloy electrocatalysts
Nature Energy (2023)