Abstract
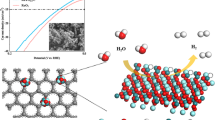
The development of acid-stable oxygen evolution reaction electrocatalysts is essential for high-performance water splitting. Here, we report an electrocatalyst with Ru-atom-array patches supported on α-MnO2 (Ru/MnO2) for the oxygen evolution reaction following a mechanism that involves only *O and *OH species as intermediates. This mechanism allows direct O–O radical coupling for O2 evolution. Ru/MnO2 shows high activity (161 mV at 10 mA cm−2) and outstanding stability with small degradation after 200 h operation, making it one of the best-performing acid-stable oxygen evolution reaction catalysts. Operando vibrational and mass spectroscopy measurements were performed to probe the reaction intermediates and gaseous products for validating the oxygen evolution reaction pathway. First-principles calculations confirmed the cooperative catalysis mechanism with a reduced energy barrier. Time-dependent elemental analysis demonstrated the occurrence of the in-situ dynamic cation exchange reaction during the oxygen evolution reaction, which is the key for triggering the reconstruction of Ru atoms into the ordered array with high durability.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Source data are provided with this paper. The data supporting the findings of this study are available within the article and its Supplementary Information or from the corresponding authors upon reasonable request.
Code availability
The software codes for Large-scale Atomic Simulation with neural network Potential and the NN potentials used within the article are available from the corresponding authors upon request or on the website http://www.lasphub.com.
References
King, L. A. et al. A non-precious metal hydrogen catalyst in a commercial polymer electrolyte membrane electrolyser. Nat. Nanotechnol. 14, 1071–1074 (2019).
Seitz, L. C. et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 353, 1011–1014 (2016).
Li, A. et al. Stable potential windows for long-term electrocatalysis by manganese oxides under acidic conditions. Angew. Chem. Int. Ed. 58, 5054–5058 (2019).
Johnson Matthey Price Charts (Johnson Matthey, accessed 15 December 2020); www.platinum.matthey.com/prices/price-charts#
Rao, R. R. et al. Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516–525 (2020).
Stoerzinger, K. A. et al. The role of Ru redox in pH-dependent oxygen evolution on rutile ruthenium dioxide surfaces. Chem 2, 668–675 (2017).
Yao, Y. et al. Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis. Nat. Catal. 2, 304–313 (2019).
Reier, T., Nong, H. N., Teschner, D., Schlögl, R. & Strasser, P. Electrocatalytic oxygen evolution reaction in acidic environments – reaction mechanisms and catalysts. Adv. Energy Mater. 7, 1601275 (2017).
Song, J. et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020).
Koper, M. T. M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 4, 2710–2723 (2013).
Rossmeisl, J., Qu, Z. W., Zhu, H., Kroes, G. J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Huang, Z.-F. et al. Strategies to break the scaling relation toward enhanced oxygen electrocatalysis. Matter 1, 1494–1518 (2019).
Hao, S. et al. Dopants fixation of ruthenium for boosting acidic oxygen evolution stability and activity. Nat. Commun. 11, 5368 (2020).
Chen, H. et al. Optimization of active sites via crystal phase, composition, and morphology for efficient low-iridium oxygen evolution catalysts. Angew. Chem. Int. Ed. 59, 19654–19658 (2020).
Shan, J. et al. Charge-redistribution-enhanced nanocrystalline Ru@IrOx electrocatalysts for oxygen evolution in acidic media. Chem 5, 445–459 (2019).
Shan, J., Ling, T., Davey, K., Zheng, Y. & Qiao, S.-Z. Transition-metal-doped RuIr bifunctional nanocrystals for overall water splitting in acidic environments. Adv. Mater. 31, 1900510 (2019).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Wang, J. et al. Amorphization activated ruthenium-tellurium nanorods for efficient water splitting. Nat. Commun. 10, 5692 (2019).
Kim, M., Park, J., Kang, M., Kim, J. Y. & Lee, S. W. Toward efficient electrocatalytic oxygen evolution: emerging opportunities with metallic pyrochlore oxides for electrocatalysts and conductive supports. ACS Cent. Sci. 6, 880–891 (2020).
Huang, Z.-F. et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329–338 (2019).
Bockris, J. O. M. Kinetics of activation controlled consecutive electrochemical reactions: anodic evolution of oxygen. J. Chem. Phys. 24, 817–827 (1956).
Song, F. et al. An unconventional iron nickel catalyst for the oxygen evolution reaction. ACS Cent. Sci. 5, 558–568 (2019).
Garrido-Barros, P., Gimbert-Suriñach, C., Matheu, R., Sala, X. & Llobet, A. How to make an efficient and robust molecular catalyst for water oxidation. Chem. Soc. Rev. 46, 6088–6098 (2017).
Roy, C. et al. Trends in activity and dissolution on RuO2 under oxygen evolution conditions: particles versus well-defined extended surfaces. ACS Energy Lett. 3, 2045–2051 (2018).
Hodnik, N. et al. New insights into corrosion of ruthenium and ruthenium oxide nanoparticles in acidic media. J. Phys. Chem. C 119, 10140–10147 (2015).
Rong, X., Parolin, J. & Kolpak, A. M. A fundamental relationship between reaction mechanism and stability in metal oxide catalysts for oxygen evolution. ACS Catal. 6, 1153–1158 (2016).
Kodera, M. et al. Reversible O–O bond scission of peroxodiiron(III) to high-spin oxodiiron(IV) in dioxygen activation of a diiron center with a bis-tpa dinucleating ligand as a soluble methane monooxygenase model. J. Am. Chem. Soc. 134, 13236–13239 (2012).
Okamura, M. et al. A pentanuclear iron catalyst designed for water oxidation. Nature 530, 465–468 (2016).
Zhang, H. et al. Enhanced interactions between gold and MnO2 nanowires for water oxidation: a comparison of different chemical and physical preparation methods. ACS Sustain. Chem. Eng. 3, 2049–2057 (2015).
Ling, T., Jaroniec, M. & Qiao, S.-Z. Recent progress in engineering the atomic and electronic structure of electrocatalysts via cation exchange reactions. Adv. Mater. 32, 2001866 (2020).
Lübke, M. et al. Transition-metal-doped α-MnO2 nanorods as bifunctional catalysts for efficient oxygen reduction and evolution reactions. ChemistrySelect 3, 2613–2622 (2018).
Morgan, D. J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 47, 1072–1079 (2015).
Liu, X. et al. Electrochemo-mechanical effects on structural integrity of Ni-rich cathodes with different microstructures in all solid-state batteries. Adv. Energy Mater. 11, 2003583 (2021).
Guo, Y. et al. Low-temperature CO2 methanation over CeO2-supported Ru single atoms, nanoclusters, and nanoparticles competitively tuned by strong metal–support interactions and H-spillover effect. ACS Catal. 8, 6203–6215 (2018).
Miao, X. et al. Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation. Nat. Commun. 10, 3809 (2019).
Cao, L. et al. Dynamic oxygen adsorption on single-atomic ruthenium catalyst with high performance for acidic oxygen evolution reaction. Nat. Commun. 10, 4849 (2019).
Binninger, T. et al. Thermodynamic explanation of the universal correlation between oxygen evolution activity and corrosion of oxide catalysts. Sci. Rep. 5, 12167 (2015).
Kötz, R., Lewerenz, H. J. & Stucki, S. XPS studies of oxygen evolution on Ru and RuO2 anodes. J. Electrochem. Soc. 130, 825–829 (1983).
Kwon, T. et al. Interfacing RuO2 with Pt to induce efficient charge transfer from Pt to RuO2 for highly efficient and stable oxygen evolution in acidic media. J. Mater. Chem. A 9, 14352–14362 (2021).
Frydendal, R., Paoli, E. A., Chorkendorff, I., Rossmeisl, J. & Stephens, I. E. L. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti-stabilized MnO2. Adv. Energy Mater. 5, 1500991 (2015).
Huang, S.-D., Shang, C., Zhang, X.-J. & Liu, Z.-P. Material discovery by combining stochastic surface walking global optimization with a neural network. Chem. Sci. 8, 6327–6337 (2017).
Shang, C., Zhang, X.-J. & Liu, Z.-P. Stochastic surface walking method for crystal structure and phase transition pathway prediction. Phys. Chem. Chem. Phys. 16, 17845–17856 (2014).
García-Mota, M. et al. Tailoring the activity for oxygen evolution electrocatalysis on rutile TiO2(110) by transition-metal substitution. ChemCatChem 3, 1607–1611 (2011).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Chang, C.-J., Chu, Y.-C., Yan, H.-Y., Liao, Y.-F. & Chen, H. M. Revealing the structural transformation of rutile RuO2 via in situ X-ray absorption spectroscopy during the oxygen evolution reaction. Dalton Trans. 48, 7122–7129 (2019).
Cheng, W. et al. Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat. Energy 4, 115–122 (2019).
Su, H. et al. Dynamic evolution of solid–liquid electrochemical interfaces over single-atom active sites. J. Am. Chem. Soc. 142, 12306–12313 (2020).
Lang, C. et al. Observation of a potential-dependent switch of water-oxidation mechanism on Co-oxide-based catalysts. Chem https://doi.org/10.1016/j.chempr.2021.03.015 (2021).
Wang, B. et al. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 13, 2200–2208 (2020).
Vivek, J. P., Berry, N. G., Zou, J., Nichols, R. J. & Hardwick, L. J. In situ surface-enhanced infrared spectroscopy to identify oxygen reduction products in nonaqueous metal–oxygen batteries. J. Phys. Chem. C 121, 19657–19667 (2017).
Tao, H. B. et al. A general method to probe oxygen evolution intermediates at operating conditions. Joule 3, 1498–1509 (2019).
Zhang, N. et al. Lattice oxygen activation enabled by high-valence metal sites for enhanced water oxidation. Nat. Commun. 11, 4066 (2020).
Pfeifer, V. et al. In situ observation of reactive oxygen species forming on oxygen-evolving iridium surfaces. Chem. Sci. 8, 2143–2149 (2017).
Liang, C. et al. Exceptional performance of hierarchical Ni–Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 13, 86–95 (2020).
Kuznetsov, D. A. et al. Tailoring lattice oxygen binding in ruthenium pyrochlores to enhance oxygen evolution activity. J. Am. Chem. Soc. 142, 7883–7888 (2020).
Wang, J. et al. Boosting the electrocatalytic activity of Co3O4 nanosheets for a Li-O2 battery through modulating inner oxygen vacancy and exterior Co3+/Co2+ ratio. ACS Catal. 7, 6533–6541 (2017).
Bao, J. et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. Int. Ed. 54, 7399–7404 (2015).
Näslund, L.-Å., Ingason, Á. S., Holmin, S. & Rosen, J. Formation of RuO(OH)2 on RuO2-based electrodes for hydrogen production. J. Phys. Chem. C 118, 15315–15323 (2014).
Liang, Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Lei, C. et al. Fe-N4 sites embedded into carbon nanofiber integrated with electrochemically exfoliated graphene for oxygen evolution in acidic medium. Adv. Energy Mater. 8, 1801912 (2018).
Xu, X., Song, F. & Hu, X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 7, 12324 (2016).
Görlin, M. et al. Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603–5614 (2016).
Huang, S.-D., Shang, C., Kang, P.-L. & Liu, Z.-P. Atomic structure of boron resolved using machine learning and global sampling. Chem. Sci. 9, 8644–8655 (2018).
Huang, S.-D., Shang, C., Kang, P.-L., Zhang, X.-J. & Liu, Z.-P. LASP: fast global potential energy surface exploration. WIREs Comput. Mol. Sci. 9, e1415 (2019).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991).
Cococcioni, M. & De Gironcoli, S. Linear response approach to the calculation of the effective interaction parameters in the LDA + U method. Phys. Rev. B 71, 035105 (2005).
García-Mota, M. et al. Importance of correlation in determining electrocatalytic oxygen evolution activity on cobalt oxides. J. Phys. Chem. C 116, 21077–21082 (2012).
Li, Y.-F. & Selloni, A. Mechanism and activity of water oxidation on selected surfaces of pure and Fe-doped NiOx. ACS Catal. 4, 1148–1153 (2014).
Shang, C. & Liu, Z. P. Stochastic surface walking method for structure prediction and pathway searching. J. Chem. Theory Comput. 9, 1838–1845 (2013).
Shang, C. & Liu, Z.-P. Constrained Broyden minimization combined with the dimer method for locating transition state of complex reactions. J. Chem. Theo. Comput. 6, 1136–1144 (2010).
Fattebert, J. L. & Gygi, F. Linear-scaling first-principles molecular dynamics with plane-waves accuracy. Phys. Rev. B 73, 115124 (2006).
Fang, Y. H. & Liu, Z. P. Mechanism and Tafel lines of electro-oxidation of water to oxygen on RuO2(110). J. Am. Chem. Soc. 132, 18214–18222 (2010).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Li, Y.-F. & Liu, Z.-P. Particle size, shape and activity for photocatalysis on titania anatase nanoparticles in aqueous surroundings. J. Am. Chem. Soc. 133, 15743–15752 (2011).
You, B., Jiang, N., Sheng, M., Bhushan, M. W. & Sun, Y. Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. ACS Catal. 6, 714–721 (2016).
Li, Y.-F. & Liu, Z.-P. Active site revealed for water oxidation on electrochemically induced δ-MnO2: role of spinel-to-layer phase transition. J. Am. Chem. Soc. 140, 1783–1792 (2018).
Li, Y.-F., Liu, Z.-P., Liu, L. & Gao, W. Mechanism and activity of photocatalytic oxygen evolution on titania anatase in aqueous surroundings. J. Am. Chem. Soc. 132, 13008–13015 (2010).
Gao, T. et al. Synthesis and properties of layered-structured Mn5O8 nanorods. J. Phys. Chem. C 114, 922–928 (2010).
Suen, N. T. et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017).
Burstein, G. T. A century of Tafel’s equation: a commemorative issue of corrosion science. Corros. Sci. 47, 2855–2856 (2005).
& Forslund, R. P. et al. Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1−xFexO4±δ Ruddlesden–Popper oxides. Nat. Commun. 9, 3150 (2018).
Naresh Kumar, T., Sivabalan, S., Chandrasekaran, N. & Phani, K. L. Synergism between polyurethane and polydopamine in the synthesis of Ni–Fe alloy monoliths. Chem. Commun. 51, 1922–1925 (2015).
Sultan, S. et al. Superb water splitting activity of the electrocatalyst Fe3Co(PO4)4 designed with computation aid. Nat. Commun. 10, 5195 (2019).
Acknowledgements
J.-H.L. appreciates the support from the Creative Materials Discovery Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (2018M3D1A1057844). X.L. acknowledges financial support from the National Natural Science Foundation of China (no. 21972163), the Fundamental Research Funds for the Central Universities, the DHU Distinguished Young Professor Program, the Development Fund for Shanghai Talents, and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning. Z.J. acknowledges the financial support from the National Natural Science Foundation of China (no. U1732267). XANES and EXAFS studies were carried out with the BL14W1 beamline at the Shanghai Synchrotron Radiation Facility (16ssrf00787). Z.-P.L. appreciates the support from National Key Research and Development Program of China (no. 2018YFA0208600). We thank Q. Liu from the University of Science and Technology of China for his helpful suggestions with synchrotron FTIR measurement and H. Zhang from the Shanghai Synchrotron Radiation Facility for his assistance in XPS analysis.
Author information
Authors and Affiliations
Contributions
C.L., X.L. and J.-H.L. conceived the project design. C.L. prepared the samples and measured their electrochemical properties. C.L., S.-H.K., D.-H.K. and S.S.S. performed the XPS, SEM and TEM characterizations. S.Y., Y.Z., X.L. and Z.J. performed the operando measurements and analysed the data. J.-L.L., Y.-F.L. and Z.-P.L. performed the DFT calculations. C.L., X.L., Z.J. and J.-H.L. wrote and revised the paper with help from all authors. W.L. helped with the manuscript revision. J.-H.L. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Sergio Rojas, Kirsten Winther and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–34, Tables 1–13, Notes 1–7 and Methods.
Supplementary Data 1
The coordinates of structure by the fixed-cell SSW-NN method of Ru-doped MnO2 (110) surface in Supplementary Fig. 20.
Supplementary Data 2
The coordinates of structure for key intermediates of the LOM pathway on the Mn site in Supplementary Fig. 21.
Source data
Source Data Fig. 3
Chronopotentiometry curves.
Source Data Fig. 5
The coordinates of structures for key intermediates of the OPM and AEM reaction pathways.
Rights and permissions
About this article
Cite this article
Lin, C., Li, JL., Li, X. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat Catal 4, 1012–1023 (2021). https://doi.org/10.1038/s41929-021-00703-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00703-0
This article is cited by
-
Tensile straining of iridium sites in manganese oxides for proton-exchange membrane water electrolysers
Nature Communications (2024)
-
Stabilization of layered lithium-rich manganese oxide for anion exchange membrane fuel cells and water electrolysers
Nature Catalysis (2024)
-
Electrocatalytic water oxidation with manganese phosphates
Nature Communications (2024)
-
Dual-site segmentally synergistic catalysis mechanism: boosting CoFeSx nanocluster for sustainable water oxidation
Nature Communications (2024)
-
Constructing regulable supports via non-stoichiometric engineering to stabilize ruthenium nanoparticles for enhanced pH-universal water splitting
Nature Communications (2024)