Abstract
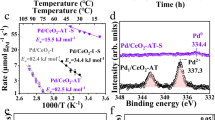
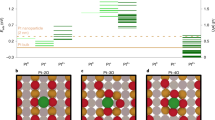
In recent years, noble metals atomically dispersed on solid oxide supports have become a frontier of heterogeneous catalysis. In pursuit of an ultimate atom efficiency, the stability of single-atom catalysts is pivotal. Here we compare two Pd/CeO2 single-atom catalysts that are active in low-temperature CO oxidation and display drastically different structural dynamics under the reaction conditions. These catalysts were obtained by conventional impregnation on hydrothermally synthesized CeO2 and one-step flame spray pyrolysis. The oxidized Pd atoms in the impregnated catalyst were prone to reduction and sintering during CO oxidation, whereas they remained intact on the surface of the Pd-doped CeO2 derived by flame spray pyrolysis. A detailed in situ characterization linked the stability of the Pd single atoms to the reducibility of the Pd–CeO2 interface and the extent of reverse oxygen spillover. To understand the chemical phenomena that underlie the metal–support interactions is crucial to the rational design of stable single-atom catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the published article (and its Supplementary Information) or available from the corresponding author upon reasonable request.
References
van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal–support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
O’Connor, N. J., Jonayat, A. S. M., Janik, M. J. & Senftle, T. P. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat. Catal. 1, 531–539 (2018).
Hackett, S. F. J. et al. High-activity, single-site mesoporous Pd/Al2O3 catalysts for selective aerobic oxidation of allylic alcohols. Angew. Chem. Int. Ed. 46, 8593–8596 (2007).
Lang, R. et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 10, 234 (2019).
Herzing, A. A., Kiely, C. J., Carley, A. F., Landon, P. & Hutchings, G. J. Identification of active gold nanoclusters on iron oxide supports for CO oxidation. Science 321, 1331–1335 (2008).
Le, Y. et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science 328, 224–228 (2010).
Parastaev, A. et al. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 3, 526–533 (2020).
Yang, X. F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Li, J., Li, X., Zhai, H. J. & Wang, L. S. Au20: A tetrahedral cluster. Science 299, 864–867 (2003).
Lykhach, Y. et al. Counting electrons on supported nanoparticles. Nat. Mater. 15, 284–288 (2016).
Campbell, C. T. Catalyst–support interactions: electronic perturbations. Nat. Chem. 4, 597–598 (2012).
Flytzani-Stephanopoulos, M. & Gates, B. C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 3, 545–574 (2012).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Yu, W.-Z. et al. Construction of active site in a sintered copper–ceria nanorod catalyst. J. Am. Chem. Soc. 141, 17548–17557 (2019).
Li, J. et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal–support interactions. J. Am. Chem. Soc. 141, 14515–14519 (2019).
DeRita, L. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Lin, J. et al. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. Sci. Eng. 38, 439–520 (1996).
Montini, T., Melchionna, M., Monai, M. & Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 116, 5987–6041 (2016).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Dvořák, F. et al. Creating single-atom Pt–ceria catalysts by surface step decoration. Nat. Commun. 7, 10801 (2016).
Sarma, B. B. et al. One-pot cooperation of single-atom Rh and Ru solid catalysts for a selective tandem olefin isomerization-hydrosilylation process. Angew. Chem. Int. Ed. 59, 5806 (2020).
Riley, C. et al. Design of effective catalysts for selective alkyne hydrogenation by doping of ceria with a single-atom promotor. J. Am. Chem. Soc. 140, 12964–12973 (2018).
Pereira-Hernández, X. I. et al. Tuning Pt–CeO2 interactions by high-temperature vapor-phase synthesis for improved reducibility of lattice oxygen. Nat. Commun. 10, 1358 (2019).
Gänzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 56, 13078–13082 (2017).
Kopelent, R. et al. Catalytically active and spectator Ce3+ in ceria-supported metal catalysts. Angew. Chem. Int. Ed. 54, 8728–8731 (2015).
Resasco, J. et al. Uniformity is key in defining structure-function relationships for atomically dispersed metal catalysts: the case of Pt/CeO2. J. Am. Chem. Soc. 142, 169–184 (2020).
Gänzler, A. M. et al. Tuning the Pt/CeO2 interface by in situ variation of the Pt particle size. ACS Catal. 8, 4800–4811 (2018).
Wang, H. et al. Surpassing the single-atom catalytic activity limit through paired Pt–O–Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 10, 3808 (2019).
Golunski, S. E. Why use platinum in catalytic converters? Platin. Met. Rev. 51, 162 (2007).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Spezzati, G. et al. Atomically dispersed Pd–O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 7, 6887–6891 (2017).
Spezzati, G. et al. CO oxidation by Pd supported on CeO2(100) and CeO2(111) facets. Appl. Catal. B 243, 36–46 (2019).
Jeong, H. et al. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 3, 368–375 (2020).
Vayssilov, G. N. et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat. Mater. 10, 310–315 (2011).
Puigdollers, A. R., Schlexer, P., Tosoni, S. & Pacchioni, G. Increasing oxide reducibility: the role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 7, 6493–6513 (2017).
Cargnello, M. et al. Control of metal nanocrystal size reveals metal–support interface role for ceria catalysts. Science 341, 771–773 (2013).
Mädler, L., Stark, W. J. & Pratsinis, S. E. Flame-made ceria nanoparticles. J. Mater. Res. 17, 1356–1362 (2002).
Koirala, R., Pratsinis, S. E. & Baiker, A. Synthesis of catalytic materials in flames: opportunities and challenges. Chem. Soc. Rev. 45, 3053 (2016).
Beniya, A. & Higashi, S. Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019).
Gröhn, A. J., Pratsinis, S. E., Sánchez-Ferrer, A., Mezzenga, R. & Wegner, K. Scale-up of nanoparticle synthesis by flame spray pyrolysis: the high-temperature particle residence time. Ind. Eng. Chem. Res. 53, 10734–10742 (2014).
Liang, H. et al. Aqueous co-precipitation of Pd-doped cerium oxide nanoparticles: chemistry, structure, and particle growth. J. Mater. Sci. 47, 299–307 (2012).
Su, Y.-Q., Filot, I. A. W., Liu, J.-X. & Hensen, E. J. M. Stable Pd-doped ceria structures for CH4 activation and CO oxidation. ACS Catal. 8, 75–80 (2018).
Hinokuma, S., Fujii, H., Okamoto, M., Ikeue, K. & Machida, M. Metallic Pd nanoparticles formed by Pd–O–Ce interaction: a reason for sintering-induced activation for CO oxidation. Chem. Mater. 22, 6183–6190 (2010).
Wang, X. et al. The synergy between atomically dispersed Pd and cerium oxide for enhanced catalytic properties. Nanoscale 9, 6643–6648 (2017).
Jeong, H., Bae, J., Han, J. W. & Lee, H. Promoting effects of hydrothermal treatment on the activity and durability of Pd/CeO2 catalysts for CO oxidation. ACS Catal. 7, 7097–7105 (2017).
Slavinskaya, E. M. et al. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B 166–167, 91–103 (2015).
Lu, Y. et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts. Nat. Catal. 2, 149–156 (2019).
Zafiris, G. S. & Gorte, R. J. Evidence for a second CO oxidation mechanism on Rh/ceria. J. Catal. 143, 86–91 (1993).
Su, Y.-Q. et al. Theoretical approach to predict the stability of supported single-atom catalysts. ACS Catal. 9, 3289–3297 (2019).
Neitzel, A. et al. Atomically dispersed Pd, Ni, and Pt species in ceria-based catalysts: principal differences in stability and reactivity. J. Phys. Chem. C 120, 9852–9862 (2016).
Boronin, A. I. et al. Investigation of palladium interaction with cerium oxide and its state in catalysts for low-temperature CO oxidation. Catal. Today 144, 201–211 (2009).
Chen, Y. et al. Well-defined palladium–ceria interfacial electronic effects trigger CO oxidation. Chem. Commun. 54, 10140–10143 (2018).
Slavinskaya, E. M. et al. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B 166–167, 91–103 (2015).
Nilsson, J. et al. Chemistry of supported palladium nanoparticles during methane oxidation. ACS Catal. 5, 2481–2489 (2015).
Su, Y. Q., Zhang, L., Muravev, V. & Hensen, E. J. M. Lattice oxygen activation in transition metal doped ceria. Chin. J. Catal. 41, 977–984 (2020).
Kato, S. et al. Quantitative depth profiling of Ce3+ in Pt/CeO2 by in situ high-energy XPS in a hydrogen atmosphere. Phys. Chem. Chem. Phys. 17, 5078–5083 (2015).
Tovt, A. et al. Ultimate dispersion of metallic and ionic platinum on ceria. J. Mater. Chem. A 7, 13019–13028 (2019).
Zhang, F. et al. In situ elucidation of the active state of Co–CeOx catalysts in the dry reforming of methane: the important role of the reducible oxide support and interactions with cobalt. ACS Catal. 8, 3550–3560 (2018).
Skála, T., Šutara, F., Prince, K. C. & Matolín, V. Cerium oxide stoichiometry alteration via Sn deposition: influence of temperature. J. Electron. Spectros. Relat. Phenom. 169, 20–25 (2009).
Acknowledgements
E.J.M.H. and V.M. acknowledge support by the Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), a NWO Gravitation program funded by the Ministry of Education, Culture and Science of the government of the Netherlands. C.E. acknowledges funding from the MICINN/FEDER RTI2018-093996-B-32 project. We thank the Diamond Light Source for time on beamline B18 under proposal SP22225. We thank the staff at the BM26A DUBBLE beamline at the ESRF (Grenoble) for the allocation of beam time under proposal 26-01-1166. We thank T. Kimpel and W. Vrijburg for help during the XAS measurements. Experiments using synchrotron radiation XPS were performed at the CIRCE beamline at ALBA Synchrotron with the collaboration of ALBA staff and CALIPSOplus (Grant 730872) funding. F. Oropeza Palacio and F. Coumans are acknowledged for the help with RPES measurements. J. Simons is acknowledged for the help with step-response experiments.
Author information
Authors and Affiliations
Contributions
V.M. and G.S. synthesized and characterized (XRD, Brunauer–Emmet–Teller and ICP) the set of ceria samples. Y.-Q.S. helped with the interpretation of the results. V.M. performed the catalytic testing, in situ NAP-XPS and DRIFTS experiments. V.M. and A.P. performed the pulsing CO chemisorption and transmission infrared spectroscopy measurements. V.M. and A.L. performed the combined XAS/WAXS study at the ESRF. V.M., A.P. and C.E. performed the in situ RPES measurements. F.-K.C. performed the high-resolution transmission electron microscopy measurements and STEM–EDX mapping. V.M., A.P., N.K. and E.J.M.H. wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–50, Notes 1–10 and Tables 1–3.
Rights and permissions
About this article
Cite this article
Muravev, V., Spezzati, G., Su, YQ. et al. Interface dynamics of Pd–CeO2 single-atom catalysts during CO oxidation. Nat Catal 4, 469–478 (2021). https://doi.org/10.1038/s41929-021-00621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00621-1