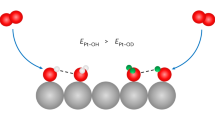
Abstract
Platinum dissolution and restructuring due to surface oxidation are primary degradation mechanisms that limit the lifetime of platinum-based electrocatalysts for electrochemical energy conversion. Here, we have studied well-defined Pt(100) and Pt(111) electrode surfaces by in situ high-energy surface X-ray diffraction, online inductively coupled plasma mass spectrometry and density functional theory calculations to elucidate the atomic-scale mechanisms of these processes. The locations of the extracted platinum atoms after Pt(100) oxidation reveal distinct differences from the Pt(111) case, which explains the different surface stability. The evolution of a specific oxide stripe structure on Pt(100) produces unstable surface atoms that are prone to dissolution and restructuring, leading to one order of magnitude higher dissolution rates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The raw X-ray data as well as the atomic coordinates of the optimized computational models have been deposited in the repository https://doi.org/10.5281/zenodo.3937672. All other data supporting the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The custom software for the analysis of the CTR data and the custom BINoculars backend for HESXRD structure factor determination are deposited in the repository https://doi.org/10.5281/zenodo.3941003. All other software used for this study is publicly available or can be obtained from the corresponding author upon reasonable request.
References
Meier, J. C. et al. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotech. 5, 44–67 (2014).
Climent, V. & Feliu, J. M. Thirty years of platinum single crystal electrochemistry. J. Solid State Electrochem. 15, 1297–1315 (2011).
Conway, B. E. & Jerkiewicz, G. Surface orientation dependence of oxide film growth at platinum single crystals. J. Electroanal. Chem. 339, 123–146 (1992).
You, H., Zurawski, D. J., Nagy, Z. & Yonco, R. M. In-situ x-ray reflectivity study of incipient oxidation of Pt(111) surface in electrolyte solutions. J. Chem. Phys. 100, 4699–4702 (1994).
Tidswell, I., Markovic, N. & Ross, P. Potential dependent surface structure of the Pt(1 1 1) electrolyte interface. J. Electroanal. Chem. 376, 119–126 (1994).
Wakisaka, M., Udagawa, Y., Suzuki, H., Uchida, H. & Watanabe, M. Structural effects on the surface oxidation processes at Pt single-crystal electrodes studied by X-ray photoelectron spectroscopy. Energy Environ. Sci. 4, 1662–1666 (2011).
Gómez-Marín, A. M. & Feliu, J. M. Oxide growth dynamics at Pt(111) in absence of specific adsorption: a mechanistic study. Electrochim. Acta 104, 367–377 (2013).
Tanaka, H. et al. Infrared reflection absorption spectroscopy of OH adsorption on the low index planes of pt. Electrocatalysis 6, 295–299 (2014).
Sugimura, F., Nakamura, M. & Hoshi, N. The oxygen reduction reaction on kinked stepped surfaces of Pt. Electrocatalysis 8, 46–50 (2016).
Huang, Y.-F., Kooyman, P. J. & Koper, M. T. M. Intermediate stages of electrochemical oxidation of single-crystalline platinum revealed by in situ Raman spectroscopy. Nat. Commun. 7, 12440 (2016).
Sugimura, F. et al. In situ observation of Pt oxides on the low index planes of Pt using surface enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 19, 27570–27579 (2017).
Furuya, N. & Shibata, M. Structural changes at various Pt single crystal surfaces with potential cycles in acidic and alkaline solutions. J. Electroanal. Chem. 467, 85–91 (1999).
Itaya, K., Sugawara, S., Sashikata, K. & Furuya, N. In situ scanning tunneling microscopy of platinum (111) surface with the observation of monatomic steps. J. Vac. Sci. Technol. A 8, 515–519 (1990).
Lopes, P. P. et al. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 6, 2536–2544 (2016).
Lopes, P. P. et al. Dynamics of electrochemical Pt dissolution at atomic and molecular levels. J. Electroanal. Chem. 819, 123–129 (2018).
Sandbeck, D. J. et al. Dissolution of platinum single crystals in acidic medium. ChemPhysChem 20, 2997–3003 (2019).
Ruge, M. et al. Structural reorganization of Pt(111) electrodes by electrochemical oxidation and reduction. J. Am. Chem. Soc. 139, 4532–4539 (2017).
Ruge, M. et al. Electrochemical oxidation of smooth and nanoscale rough Pt(111): an in situ surface X-ray scattering study. J. Electrochem. Soc. 164, H608–H614 (2017).
Jacobse, L., Huang, Y.-F., Koper, M. T. M. & Rost, M. J. Correlation of surface site formation to nanoisland growth in the electrochemical roughening of Pt(111). Nat. Mater. 17, 277–282 (2018).
Arulmozhi, N., Esau, D., Lamsal, R. P., Beauchemin, D. & Jerkiewicz, G. Structural transformation of monocrystalline platinum electrodes upon electro-oxidation and electro-dissolution. ACS Catal. 8, 6426–6439 (2018).
Topalov, A. A. et al. Dissolution of platinum: limits for the deployment of electrochemical energy conversion? Angew. Chem. Int. Ed. 51, 12613–12615 (2012).
Gómez-Marín, A. M. & Feliu, J. M. Pt(111) surface disorder kinetics in perchloric acid solutions and the influence of specific anion adsorption. Electrochim. Acta 82, 558–569 (2012).
Drnec, J. et al. Initial stages of Pt(111) electrooxidation: dynamic and structural studies by surface X-ray diffraction. Electrochim. Acta 224, 220–227 (2017).
Drnec, J., Harrington, D. & Magnussen, O. Electrooxidation of Pt(111) in acid solution. Curr. Opin. Electrochem. 4, 69–75 (2017).
Liu, Y., Barbour, A., Komanicky, V. & You, H. X-ray crystal truncation rod studies of surface oxidation and reduction on Pt(111). J. Phys. Chem. C. 120, 16174–16178 (2016).
Fantauzzi, D., Mueller, J. E., Sabo, L., van Duin, A. C. T. & Jacob, T. Surface buckling and subsurface oxygen: atomistic insights into the surface oxidation of Pt(111). ChemPhysChem 16, 2797–2802 (2015).
Eslamibidgoli, M. J. & Eikerling, M. H. Atomistic mechanism of Pt extraction at oxidized surfaces: insights from DFT. Electrocatalysis 7, 345–354 (2016).
Gu, Z. & Balbuena, P. B. Chemical environment effects on the atomic oxygen absorption into Pt(111) subsurfaces. J. Phys. Chem. C. 111, 17388–17396 (2007).
Rodes, A., Zamakhchari, M. A., El Achi, K. & Clavilier, J. Electrochemical behaviour of Pt(100) in various acidic media: part I. On a new voltammetric profile of Pt(100) in perchloric acid and effects of surface defects. J. Electroanal. Chem. 305, 115–129 (1991).
Gustafson, J. et al. High-energy surface X-ray diffraction for fast surface structure determination. Science 343, 758–761 (2014).
Devarajan, S. P., Hinojosa, J. A. & Weaver, J. F. STM study of high-coverage structures of atomic oxygen on Pt(1 1 1): p(2 × 1) and Pt oxide chain structures. Surf. Sci. 602, 3116–3124 (2008).
Van Spronsen, M. A., Frenken, J. W. & Groot, I. M. Observing the oxidation of platinum. Nat. Commun. 8, 429 (2017).
Xing, L., Jerkiewicz, G. & Beauchemin, D. Ion exchange chromatography coupled to inductively coupled plasma mass spectrometry for the study of Pt electro-dissolution. Anal. Chim. Acta 785, 16–21 (2013).
Cherevko, S., Kulyk, N. & Mayrhofer, K. J. Durability of platinum-based fuel cell electrocatalysts: dissolution of bulk and nanoscale platinum. Nano Energy 29, 275–298 (2016).
Cherevko, S., Topalov, A. A., Zeradjanin, A. R., Keeley, G. P. & Mayrhofer, K. J. J. Temperature-dependent dissolution of polycrystalline platinum in sulfuric acid electrolyte. Electrocatalysis 5, 235–240 (2014).
Geiger, S. Stability Investigations of Iridium-Based Catalysts Towards Acidic Water Splitting. Dissertation, Ruhr-Universität Bochum (2018).
Lamsal, R. P., Jerkiewicz, G. & Beauchemin, D. Enhancement of the capabilities of inductively coupled plasma mass spectrometry using monosegmented flow analysis. Anal. Chem. 90, 13842–13847 (2018).
Sandbeck, D. J. S. On the Dissolution of Platinum: From Fundamental to Advanced Catalytic Materials. Dissertation, Friedrich-Alexander-Universität Erlangen-Nürnberg (2020).
Magnussen, O. M., Krug, K., Ayyad, A. H. & Stettner, J. In situ diffraction studies of electrode surface structure during gold electrodeposition. Electrochim. Acta 53, 3449–3458 (2008).
Drnec, J. et al. Pt oxide and oxygen reduction at Pt(111) studied by surface X-ray diffraction. Electrochem. Commun. 84, 50–52 (2017).
Ashiotis, G. et al. The fast azimuthal integration Python library: PyFAI. J. Appl. Crystallogr. 48, 510–519 (2015).
Busing, W. R. & Levy, H. A. Angle calculations for 3- and 4-circle X-ray and neutron diffractometers. Acta Crystallogr. 22, 457–464 (1967).
Wang, J., Ocko, B., Davenport, A. & Isaacs, H. In situ x-ray-diffraction and -reflectivity studies of the Au(111)/electrolyte interface: Reconstruction and anion adsorption. Phys. Rev. B 46, 10321–10338 (1992).
Roobol, S., Onderwaater, W., Drnec, J., Felici, R. & Frenken, J. BINoculars: data reduction and analysis software for two-dimensional detectors in surface X-ray diffraction. J. Appl. Crystallogr. 48, 1324–1329 (2015).
Vlieg, E. Integrated intensities using a six-circle surface X-ray diffractometer. J. Appl. Crystallogr. 30, 532–543 (1997).
Drnec, J. et al. Integration techniques for surface X-ray diffraction data obtained with a two-dimensional detector. J. Appl. Crystallogr. 47, 365–377 (2014).
Feidenhans’l, R. Surface structure determination by X-ray diffraction. Surf. Sci. Rep. 10, 105–188 (1989).
Press, W. H., Teukolsky, S. A., Vettering, W. T. & Flannery, B. P.Numerical Recipes in C: The Art of Scientific Computing 2nd edn, (Cambridge Univ. Press, 1992).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Lide, D. R. (ed) CRC Handbook of Chemistry and Physics (CRC Press, 2005).
Calle-Vallejo, F., de Morais, R., Illas, F., Loffreda, D. & Sautet, P. Affordable estimation of solvation contributions to the adsorption energies of oxygenates on metal nanoparticles. J. Phys. Chem. C. 123, 5578–5582 (2019).
He, Z.-D., Hanselman, S., Chen, Y.-X., Koper, M. T. M. & Calle-Vallejo, F. Importance of solvation for the accurate prediction of oxygen reduction activities of Pt-based electrocatalysts. J. Phys. Chem. Lett. 8, 2243–2246 (2017).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 10, 3722–3730 (2008).
Janthon, P. et al. Bulk properties of transition metals: a challenge for the design of universal density functionals. J. Chem. Theory Comput. 10, 3832–3839 (2014).
Bizzotto, F. et al. Examining the structure sensitivity of the oxygen evolution reaction on Pt single-crystal electrodes: a combined experimental and theoretical study. ChemPhysChem 20, 3154–3162 (2019).
Acknowledgements
We acknowledge the European Synchrotron Radiation Facility for provision of SXRD facilities, and H. Isern and T. Dufrane for their help with the SXRD experiments. Funding is acknowledged from the NSERC (grant no. RGPIN-2017-04045) and Deutsche Forschungsgemeinschaft (grant nos. MA 1618/23 and CH 1763/5-1). F.C.-V acknowledges funding from Spanish MICIUN RTI2018-095460-B-I00 and María de Maeztu MDM-2017-0767 grants, and thanks RES for supercomputing time at SCAYLE (projects QS-2019-3-0018, QS-2019-2-0023, and QCM-2019-1-0034) and MareNostrum (project QS-2020-1-0012). The use of supercomputing facilities at SURFsara was sponsored by NWO Physical Sciences, with financial support by NWO.
Author information
Authors and Affiliations
Contributions
T.F., J.D., N.S., M.R., D.A.H. and O.M.M. designed and performed the SXRD experiments. T.F. analysed the SXRD data. D.J.S.S. and S.C. designed and performed the SFC-ICP-MS experiments. D.J.S.S. analysed the SFC-ICP-MS data. F.C.-V. performed the DFT calculations. T.F., J.D., D.A.H., D.J.S.S., S.C., F.C.-V. and O.M.M. were involved in the interpretation of the results and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cyclic voltammograms measured in the electrochemical cell used for the Surface X-ray Diffraction measurements.
Cyclic voltammograms of (a) Pt(100) and (b) Pt(111) in 0.1 M HClO4 with a scan rate of 50 mV/s.
Extended Data Fig. 2 Crystal truncation rods of Pt(100) at 0.12 V prior to surface oxidation and after oxide reduction.
Crystal truncation rods (CTR) of the pristine Pt(100) surface after sample preparation and the CTRs of the roughened Pt(100) surface after oxide formation at 1.17 V and subsequent oxide reduction at 0.12 V. The grey lines indicate the CTRs of a bulk terminated Pt(100) surface. The decrease of the CTR structure factor after surface oxidation can be attributed to the formation of adatoms Ptad and vacancies in the Pt1 layer. Best fits with a quantitative model (solid blue line) that includes these surface defects result in a Ptad coverage of 0.07 ML.
Extended Data Fig. 3 Crystal truncation rods (CTR) and corresponding CTR fits of Pt(100) close to and in the region of oxide formation.
CTRs of Pt(100) at a potential slightly negative (0.95 V) and three potentials positive (1.07, 1.12 and 1.17 V) of the Oads peak in the cyclic voltammogram (Fig. 1b, Extended Data Fig. 1). Solid lines are the corresponding CTR fits. The CTRs for the different potentials are offset to each other by a factor 10 and shown together with the CTR fits of the smooth surface at 0.95 V (grey lines). Details on the CTR fits are given in the Supplementary Note 3 and the corresponding structural parameters are given in Supplementary Table 4.
Extended Data Fig. 4 Gibbs energy for the first Pt extraction and the subsequent dissolution of Pt.
(a) Oxygen coverage θO dependent Gibbs energy ΔG for the extraction of the first atom on Pt(111) and Pt(100). (b) ΔG for the dissolution of the extracted atom after first extraction on Pt(111) and first, second and third extraction on Pt(100). The correspondence between the oxygen coverage θO and potential U is in the inset of (b).
Extended Data Fig. 5 Pourbaix diagrams for O adsorption.
Pourbaix diagram of (a) Pt(111) and (b) Pt(100). The dashed line represents the oxygen reduction reaction (O2 + 4(H+ + e−) → 2H2O).
Extended Data Fig. 6 Additional views of the lowest-energy structures in the process of Pt extraction.
(a) Top view of the Pt extraction process on Pt(111). (b) Side view of the Pt extraction process on Pt(100).
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Tables 1–6, Notes 1–3 and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Averaged CTR structure factors.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Averaged CTR structure factors.
Source Data Extended Data Fig. 3
Unprocessed CTR structure factors.
Rights and permissions
About this article
Cite this article
Fuchs, T., Drnec, J., Calle-Vallejo, F. et al. Structure dependency of the atomic-scale mechanisms of platinum electro-oxidation and dissolution. Nat Catal 3, 754–761 (2020). https://doi.org/10.1038/s41929-020-0497-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0497-y
This article is cited by
-
Tailoring the active site for the oxygen evolution reaction on a Pt electrode
Communications Chemistry (2022)
-
1D PtCo nanowires as catalysts for PEMFCs with low Pt loading
Science China Materials (2022)