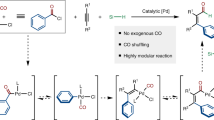
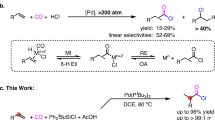
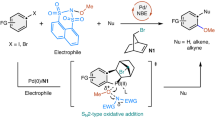
Abstract
Palladium-catalysed oxidative carbonylation using oxygen as the oxidant is an economical approach; however, the gas mixture of CO and air has an explosive limit of 12.5–74.0% that could hamper extensive application of this process. Herein we report an electrochemical aminocarbonylation of alkynes under atmospheric pressure in an undivided cell without an external oxidant. The transformation has a broad substrate scope (83 examples) that involves primary amines and ammonium salts. Furthermore, mechanistic studies through cyclic voltammetry, in situ infrared and quick-scanning X-ray absorption fine structure spectroscopy reveal the reasons for this protocol proceeding smoothly under electrochemical conditions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data including experimental procedures and compound characterization are available within the paper and its Supplementary Information. All other data are available from the authors on reasonable request.
References
Barnard, C. F. J. Palladium-catalyzed carbonylation—a reaction come of age. Organometallics 27, 5402–5422 (2008).
Brennführer, A., Neumann, H. & Beller, M. Palladium-catalyzed carbonylation reactions of aryl halides and related compounds. Angew. Chem. Int. Ed. 48, 4114–4133 (2009).
Shen, C. & Wu, X.-F. Palladium-catalyzed carbonylative multicomponent reactions. Chem. Eur. J. 23, 2973–2987 (2017).
Sumino, S., Fusano, A., Fukuyama, T. & Ryu, I. Carbonylation reactions of alkyl iodides through the interplay of carbon radicals and Pd catalysts. Acc. Chem. Res. 47, 1563–1574 (2014).
Kiss, G. Palladium-catalyzed reppe carbonylation. Chem. Rev. 101, 3435–3456 (2001).
Li, S., Chen, G., Feng, C.-G., Gong, W. & Yu, J.-Q. Ligand-enabled γ-C–H olefination and carbonylation: construction of β-quaternary carbon centers. J. Am. Chem. Soc. 136, 5267–5270 (2014).
Liu, C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015).
Liu, Q., Zhang, H. & Lei, A. Oxidative carbonylation reactions: organometallic compounds (R–M) or hydrocarbons (R–H) as nucleophiles. Angew. Chem. Int. Ed. 50, 10788–10799 (2011).
Willcox, D. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Wu, X.-F., Neumann, H. & Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 6, 229–241 (2013).
Carl, Y. & William, B. Matheson Gas Data Book (McGraw-Hill Professional, 2001).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Frontana-Uribe, B. A., Little, R. D., Ibanez, J. G., Palma, A. & Vasquez-Medrano, R. Organic electrosynthesis: a promising green methodology in organic chemistry. Green. Chem. 12, 2099–2119 (2010).
Huang, X., Zhang, Q., Lin, J., Harms, K. & Meggers, E. Electricity-driven asymmetric Lewis acid catalysis. Nat. Catal. 2, 34–40 (2019).
Jiang, Y., Xu, K. & Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 118, 4485–4540 (2017).
Jutand, A. Contribution of electrochemistry to organometallic catalysis. Chem. Rev. 108, 2300–2347 (2008).
Sperry, J. B. & Wright, D. L. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev. 35, 605–621 (2006).
Tang, S., Liu, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem 4, 27–45 (2018).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Yoshida, J.-i, Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Chiarotto, I. & Carelli, I. Palladium-catalyzed electrochemical carbonylation of alkynes under very mild conditions. Synth. Commun. 32, 881–886 (2002).
Ma, C., Fang, P. & Mei, T.-S. Recent advances in C–H functionalization using electrochemical transition metal catalysis. ACS Catal. 8, 7179–7189 (2018).
Moeller, K. D. Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 118, 4817–4833 (2018).
Sauermann, N., Meyer, T. H., Qiu, Y. & Ackermann, L. Electrocatalytic C–H activation. ACS Catal. 8, 7086–7103 (2018).
Xu, F., Li, Y.-J., Huang, C. & Xu, H.-C. Ruthenium-catalyzed electrochemical dehydrogenative alkyne annulation. ACS Catal. 8, 3820–3824 (2018).
Zhang, L. et al. Photoelectrocatalytic arene C–H amination. Nat. Catal. 2, 366–373 (2019).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Gabriele, B., Salerno, G., Veltri, L. & Costa, M. Synthesis of 2-ynamides by direct palladium-catalyzed oxidative aminocarbonylation of alk-1-ynes. J. Organomet. Chem. 622, 84–88 (2001).
Gadge, S. T., Khedkar, M. V., Lanke, S. R. & Bhanage, B. M. Oxidative aminocarbonylation of terminal alkynes for the synthesis of alk-2-ynamides by using palladium-on-carbon as efficient, heterogeneous, phosphine-free, and reusable catalyst. Adv. Synth. Catal. 354, 2049–2056 (2012).
Hughes, N. L., Brown, C. L., Irwin, A. A., Cao, Q. & Muldoon, M. J. Palladium(ii)-catalysed aminocarbonylation of terminal alkynes for the synthesis of 2-ynamides: addressing the challenges of solvents and gas mixtures. ChemSusChem 10, 675–680 (2017).
Veltri, L. et al. A palladium iodide-catalyzed oxidative aminocarbonylation–heterocyclization approach to functionalized benzimidazoimidazoles. J. Org. Chem. 83, 1680–1685 (2018).
Zhang, C., Liu, J. & Xia, C. Palladium–N-heterocyclic carbene (NHC)-catalyzed synthesis of 2-ynamides via oxidative aminocarbonylation of alkynes with amines. Catal. Sci. Technol. 5, 4750–4754 (2015).
Choi, Y.-S. et al. Ionic liquids as benign catalysts for the carbonylation of amines to formamides. Appl. Catal. A Gen. 404, 87–92 (2011).
Gerack, C. J. & McElwee-White, L. Oxidative carbonylation of amines to formamides using NaIO4. Chem. Commun. 48, 11310–11312 (2012).
Li, X. et al. N-Heterocyclic carbene catalyzed direct carbonylation of dimethylamine. Chem. Commun. 47, 7860–7862 (2011).
Preiß, S. et al. Structure and reactivity of a mononuclear gold(ii) complex. Nat. Chem. 9, 1249–1255 (2017).
Yuan, N. et al. Probing the evolution of palladium species in Pd@MOF catalysts during the heck coupling reaction: an operando X‑ray absorption spectroscopy study. J. Am. Chem. Soc. 140, 8206–8217 (2018).
Venderbosch, B. et al. Spectroscopic investigation of the activation of a chromium-pyrrolyl ethene trimerization catalyst. ACS Catal. 9, 1197–1210 (2019).
Tran, B. et al. Operando XAFS studies on Rh(CAAC)-catalyzed arene hydrogenation. ACS Catal. 9, 4106–4114 (2019).
Yi, H. et al. Unravelling the hidden link of lithium halides and application in the synthesis of organocuprates. Nat. Commun. 8, 14794 (2017).
Bai, R. et al. Cu(ii)/Cu(i)-synergistic cooperation to lead the alkyne C–H activation. J. Am. Chem. Soc. 136, 16760–16763 (2014).
Zhang, G. et al. Direct observation of reduction of Cu(ii) to Cu(i) by terminal alkynes. J. Am. Chem. Soc. 136, 924–926 (2014).
Izawa, Y., Shimizu, I. & Yamamoto, A. Palladium-catalyzed oxidative carbonylation of 1-alkynes into 2-alkynoates with molecular oxygen as oxidant. B. Chem. Soc. Jpn. 77, 2033–2045 (2004).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 21520102003, 21702152), the 973 Program (grant no. 2012CB725302), the CAS Interdisciplinary Innovation Team and the Hubei Province Natural Science Foundation of China (grant no. 2017CFA010). The Program of Introducing Talents of Discipline to Universities of China (111 Program) is also appreciated. X-ray absorption spectroscopy analysis was performed at the National Synchrotron Radiation Research Center (44A in Taiwan Photon Source). We would like to thank O. Muller from Bergische University Wuppertal for providing JAQ Analyzes QEXAFS software for analysing Quick-XAFS data. We would like to thank G. Li from State Utah University and S. Tang from the Weizmann Institute of Science for advising on the manuscript.
Author information
Authors and Affiliations
Contributions
A.L., Y.-H.C. and L.Z. contributed to the conception and design of the experiments. H.L., Jingcheng H., D.Z., Jiayu H., P.P., S.W., R.S., J.P., C.-W.P., J.-L.C., J.-F.L. and H.Z. performed the experiments. L.Z., Y.-H.C. and A.L. co-wrote the manuscript and all authors contributed to data analysis and scientific discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–6, Figs. 1–36 and references.
Rights and permissions
About this article
Cite this article
Zeng, L., Li, H., Hu, J. et al. Electrochemical oxidative aminocarbonylation of terminal alkynes. Nat Catal 3, 438–445 (2020). https://doi.org/10.1038/s41929-020-0443-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-0443-z
This article is cited by
-
Asymmetric-waveform alternating current-promoted silver catalysis for C–H phosphorylation
Nature Synthesis (2023)
-
Palladium-catalyzed asymmetric allylic 4-pyridinylation via electroreductive substitution reaction
Nature Communications (2022)
-
Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water
Nature Communications (2021)