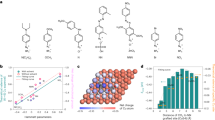
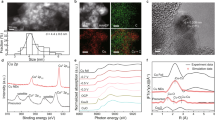
Abstract
Electrochemical conversion of CO2 into value-added chemicals holds promise to enable the transition to carbon neutrality. Enhancing selectivity for a specific hydrocarbon product is challenging, however, due to numerous possible reaction pathways of CO2 electroreduction. Here we present a Cu–polyamine hybrid catalyst, developed through co-electroplating, that significantly increases the selectivity for ethylene production. The Faradaic efficiency for ethylene production is 87% ± 3% at −0.47 V versus reversible hydrogen electrode, with full-cell energetic efficiency reaching 50% ± 2%. Raman measurements indicate that the polyamine entrained on the Cu electrode results in higher surface pH, higher CO content and higher stabilization of intermediates compared with entrainment of additives containing little or no amine functionality. More broadly, this work shows that polymer incorporation can alter surface reactivity and lead to enhanced product selectivity at high current densities.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Montoya, J. H. et al. Materials for solar fuels and chemicals. Nat. Mater. 16, 70–81 (2016).
Hepburn, C. et al. The technological and economic prospects for CO2 utilization and removal. Nature 575, 87–97 (2019).
Hori, Y., Kikuchi, K., Murata, A. & Suzuki, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 15, 897–898 (1986).
Hori, Y., Wakebe, H., Tsukamoto, T. & Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 39, 1833–1839 (1994).
Luc, W. et al. SO2-induced selectivity change in CO2 electroreduction. J. Am. Chem. Soc. 141, 9902–9909 (2019).
Zhang, H. et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane. Nat. Commun. 10, 3340 (2019).
Yin, Z. et al. Cu3N nanocubes for selective electrochemical reduction of CO2 to ethylene. Nano Lett. 19, 8658–8663 (2019).
Wang, Y. et al. Copper nanocubes for CO2 reduction in gas diffusion electrodes. Nano Lett. 19, 8461–8468 (2019).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Ma, S. et al. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu–Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 139, 47–50 (2017).
Chen, X. et al. Controlling speciation during CO2 reduction on Cu-alloy electrodes. ACS Catal. 10, 672–682 (2019).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Hoang, T. T. H. et al. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 7, 3313–3321 (2017).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2019).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Weekes, D. M. et al. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Schmitt, K. G. & Gewirth, A. A. In situ surface-enhanced Raman spectroscopy of the electrochemical reduction of carbon dioxide on silver with 3,5-diamino-1,2,4-triazole. J. Phys. Chem. C 118, 17567–17576 (2014).
Widrig, C. A., Chung, C. & Porter, M. D. The electrochemical desorption of n-alkanethiol monolayers from polycrystalline Au and Ag electrodes. J. Electroanal. Chem. 310, 335–359 (1991).
Tsakova, V. et al. Electrochemical incorporation of copper in polyaniline layers. Electrochim. Acta 46, 4213–4222 (2001).
Ahn, S. et al. Poly-amide modified copper foam electrodes for enhanced electrochemical reduction of carbon dioxide. ACS Catal. 8, 4132–4142 (2018).
Mingqi, Z., Li, S. & M., C. R. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 120, 4877–4878 (1998).
Mohtasebi, A. et al. Interfacial charge transfer between phenyl-capped aniline tetramer films and iron oxide surfaces. J. Phys. Chem. C. 120, 29248–29263 (2016).
Sylvestre, J.-P. et al. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B 108, 16864–16869 (2004).
Schouten, K. J. P. et al. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2, 1902–1909 (2011).
Kortlever, R. et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Jiang, H. et al. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 12, 322–333 (2019).
Khurram, A. et al. Promoting amine-activated electrochemical CO2 conversion with alkali salts. J. Phys. Chem. C 123, 18222–18231 (2019).
Kortunov, P. V., Siskin, M., Paccagnini, M. & Thomann, H. CO2 reaction mechanisms with hindered alkanolamines: control and promotion of reaction pathways. Energy Fuels 30, 1223–1236 (2016).
Deng, Y. et al. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of Cuiii oxides as catalytically active species. ACS Catal. 6, 2473–2481 (2016).
Jiang, S., Klingan, K., Pasquini, C. & Dau, H. New aspects of operando Raman spectroscopy applied to electrochemical CO2 reduction on Cu foams. J. Chem. Phys. 150, 041718 (2019).
Frantz, J. D. Raman spectra of potassium carbonate and bicarbonate aqueous fluids at elevated temperatures and pressures: comparison with theoretical simulations. Chem. Geol. 152, 211–225 (1998).
Akemann, W. & Otto, A. The effect of atomic scale surface disorder on bonding and activation of adsorbates: vibrational properties of CO and CO2 on copper. Surf. Sci. 287, 104–109 (1993).
Gunathunge, C. M. et al. Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017).
Chernyshova, I. V., Somasundaran, P. & Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl Acad. Sci. USA 115, E9261–E9270 (2018).
Firet, N. J. & Smith, W. A. Probing the reaction mechanism of CO2 electroreduction over Ag films via operando infrared spectroscopy. ACS Catal. 7, 606–612 (2016).
Davis, A. R. & Oliver, B. G. A vibrational-spectroscopic study of the species present in the CO2–H2O system. J. Solut. Chem. 1, 329–339 (1972).
Klitzing, R. & Moehwald, H. Proton concentration profile in ultrathin polyelectrolyte films. Langmuir 11, 3554–3559 (1995).
Zhang, Y., Tsitkov, S. & Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade. Nat. Commun. 7, 13982 (2016).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Varela, A. S., Kroschel, M., Reier, T. & Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: the effect of the electrolyte concentration and the importance of the local pH. Catal. Today 260, 8–13 (2016).
Schouten, K. J. P., Pérez Gallent, E. & Koper, M. T. M. The influence of pH on the reduction of CO and CO2 to hydrocarbons on copper electrodes. J. Electroanal. Chem. 716, 53–57 (2014).
Raciti, D., Mao, M., Park, J. H. & Wang, C. Local pH effect in the CO2 reduction reaction on high-surface-area copper electrocatalysts. J. Electrochem. Soc. 165, F799–F804 (2018).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Lee, J. et al. Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 31, 244–250 (2019).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Tomas, M. et al. Modification of gas diffusion layers properties to improve water management. Mater. Renew. Sustain. Energy 6, 20 (2017).
Yu, S. et al. Study on hydrophobicity loss of the gas diffusion layer in PEMFCs by electrochemical oxidation. RSC Adv. 4, 3852–3856 (2014).
Verma, S. et al. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Acknowledgements
The authors gratefully acknowledge the support of the International Institute for Carbon Neutral Energy Research (WPI-I2CNER), sponsored by the Japanese Ministry of Education, Culture, Sports, Science and Technology. D.A.H., U.O.N. and P.J.A.K. gratefully acknowledge Shell’s New Energy Research and Technology (NERT) programme for providing funding. J.C. and S.C.Z. acknowledge support of the National Science Foundation (NSF CHE-1709718). We thank the School of Chemical Sciences, University of Illinois Mass Spectrometry Laboratory (especially F. Sun and X. Mao) for performing gas chromatography mass spectrometry measurements. We thank R.T. Haasch for performing XPS and the School of Chemical Sciences, University of Illinois Machine Shop for their help in designing the in situ flow cell for the Raman measurements. We also thank the School of Chemical Sciences, University of Illinois NMR Laboratory for their help with the NMR measurements.
Author information
Authors and Affiliations
Contributions
X.C. and N.M.A. prepared the Cu–Pi electrodes and performed the electrochemistry experiments. J.C. synthesized the polymers and performed the NMR experiments. X.C. conducted the SEM and XRD experiments. X.C. and D.A.H. carried out the Raman measurements. R.Z. analysed the XPS data. U.O.N. prepared the anode electrodes. K.E.M. did the contact angle measurements. A.A.G., S.C.Z. and P.J.A.K. conceived the project and supervised the research work. X.C., J.C. and A.A.G. wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–30 and Tables 1–5.
Rights and permissions
About this article
Cite this article
Chen, X., Chen, J., Alghoraibi, N.M. et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat Catal 4, 20–27 (2021). https://doi.org/10.1038/s41929-020-00547-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-020-00547-0
This article is cited by
-
A surface strategy boosting the ethylene selectivity for CO2 reduction and in situ mechanistic insights
Nature Communications (2024)
-
Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction
Nature Communications (2024)
-
Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization
Nature Energy (2024)
-
Molecular understanding of the critical role of alkali metal cations in initiating CO2 electroreduction on Cu(100) surface
Nature Communications (2024)
-
Acidic CO2-to-HCOOH electrolysis with industrial-level current on phase engineered tin sulfide
Nature Communications (2023)