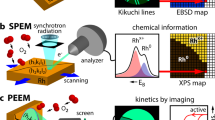
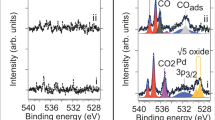
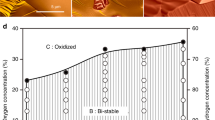
Abstract
Analytical methods that provide direct real-space information about the dynamics of catalysed reactions often require simplified model systems and operate under high-vacuum conditions. There is thus a strong need for the development of methods that enable observation of active catalysts under relevant working conditions. Here, in situ scanning electron microscopy is employed to study reaction dynamics and structure–activity correlations on surfaces. High sensitivity to changes in the work function and surface composition enables the detection of monolayers of adsorbed molecular species on metal surfaces, which is used here to visualize catalytic NO2 hydrogenation on platinum. The initiation of reactive behaviours and propagation of reaction fronts, as well as the spillover of activated species revealed in real-time and across a large pressure range, demonstrate the power of in situ scanning electron microscopy as a surface science tool in the study of gas-phase- and temperature-induced processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All recorded images and data that support the findings of this study are available from the corresponding author on reasonable request.
References
Kuwauchi, Y., Yoshida, H., Akita, T., Haruta, M. & Takeda, S. Intrinsic catalytic structure of gold nanoparticles supported on TiO2. Angew. Chem. Int. Ed. 51, 7729–7733 (2012).
Roobol, S. B. et al. The reactor AFM: non-contact atomic force microscope operating under high-pressure and high-temperature catalytic conditions. Rev. Sci. Instrum. 86, 033706 (2015).
Hendriksen, B. L. M. et al. The role of steps in surface catalysis and reaction oscillations. Nat. Chem. 2, 730–734 (2010).
Mom, R. V. et al. Simultaneous scanning tunneling microscopy and synchrotron X-ray measurements in a gas environment. Ultramicroscopy 182, 233–242 (2017).
Genty, E., Jacobs, L., Visart de Bocarmé, T. & Barroo, C. Dynamic processes on gold-based catalysts followed by environmental microscopies. Catalysts 7, 134 (2017).
Takayanagi, K. High resolution surface study by in-situ UHV transmission electron microscopy. Ultramicroscopy 8, 145–161 (1982).
Thomas, J. M. Reflections on the value of electron microscopy in the study of heterogeneous catalysts. Proc. R. Soc. A 473, 20160714 (2017).
Vendelbo, S. B. et al. Visualization of oscillatory behaviour of Pt nanoparticles catalysing CO oxidation. Nat. Mater. 13, 884–890 (2014).
Nettesheim, S., von Oertzen, A., Rotermund, H. H. & Ertl, G. Reaction diffusion patterns in the catalytic CO-oxidation on Pt(110): front propagation and spiral waves. J. Chem. Phys. 98, 9977–9985 (1993).
von Boehn, B. & Imbihl, R. Large amplitude excitations traveling along the interface in bistable catalytic methanol oxidation on Rh(110). Phys. Chem. Chem. Phys. 19, 18487–18493 (2017).
Suchorski, Y. & Rupprechter, G. Local reaction kinetics by imaging. Surf. Sci. 643, 52–58 (2016).
Gorodetskii, V. V., Block, J. H. & Drachsel, W. Isothermal oscillations of the hydrogen-oxidation reaction on platinum: investigations in the field electron and field ion microscope. Appl. Surf. Sci. 76–77, 129–135 (1994).
Visart de Bocarmé, T. & Kruse, N. Field emission techniques for studying surface reactions: applying them to NO–H2 interaction with Pd tips. Ultramicroscopy 111, 376–380 (2011).
Franz, T. et al. Catalytic CO oxidation on Pt under near ambient pressure: a NAP-LEEM study. Ultramicroscopy 200, 73–78 (2019).
Sachs, C., Hildebrand, M., Völkening, S., Wintterlin, J. & Ertl, G. Spatiotemporal self-organization in a surface reaction: from the atomic to the mesoscopic scale. Science 293, 1635–1638 (2001).
van Spronsen, M. A., Frenken, J. W. M. & Groot, I. M. N. Observing the oxidation of platinum. Nat. Commun. 8, 429 (2017).
Dicke, J., Rotermund, H.-H. & Lauterbach, J. Ellipsomicroscopy for surface imaging: contrast mechanism, enhancement, and application to CO oxidation on Pt(110). J. Opt. Soc. Am. A 17, 135–141 (2000).
Danilatos, G. D. Review and outline of environmental SEM at present. J. Microsc. 162, 391–402 (1990).
Millar, G. J., Nelson, M. L. & Uwins, P. J. R. In situ imaging of catalytic etching on silver during methanol oxidation conditions by environmental scanning electron microscopy. J. Catal. 169, 143–156 (1997).
Fitzek, H., Schroettner, H., Wagner, J., Hofer, F. & Rattenberger, J. High-quality imaging in environmental scanning electron microscopy—optimizing the pressure limiting system and the secondary electron detection of a commercially available ESEM. J. Microsc. 262, 85–91 (2016).
Greiner, M. T. et al. The oxidation of copper catalysts during ethylene epoxidation. Phys. Chem. Chem. Phys. 17, 25073–25089 (2015).
Poitel, S., Wang, Z.-J., Willinger, M.-G. & Hébert, C. In-situ observation of Co–Ce coated metallic interconnect oxidation combined with high-resolution post exposure. Anal. ECS Trans. 78, 1615–1632 (2017).
Hieke, S. W. et al. On pinning–depinning and microkink-flow in solid state dewetting: insights by in-situ ESEM on Al thin films. Acta Mater. 165, 153–163 (2019).
Huang, X., Wang, Z.-J., Weinberg, G., Meng, W.-M. & Willinger, M.-G. In situ scanning electron microscopy observation of growth kinetics and catalyst splitting in vapor–liquid–solid growth of nanowires. Adv. Funct. Mater. 25, 5979–5987 (2015).
Huang, X. et al. In situ formation of crystallographically oriented semiconductor nanowire arrays via selective vaporization for optoelectronic applications. Adv. Mater. 28, 7603–7612 (2016).
Wang, Z.-J. et al. Direct observation of graphene growth and associated copper substrate dynamics by in situ scanning electron microscopy. ACS Nano 9, 1506–1519 (2015).
Blume, R. et al. The influence of intercalated oxygen on the properties of graphene on polycrystalline Cu under various environmental conditions. Phys. Chem. Chem. Phys. 16, 25989–26003 (2014).
Wang, Z.-J. et al. Stacking sequence and interlayer coupling in few-layer graphene revealed by in situ imaging. Nat. Commun. 7, 13256 (2016).
Voss, C. & Kruse, N. Oscillatory behavior in the catalytic reduction of NO and NO2 with hydrogen on Pt field emitter tips. Appl. Surf. Sci. 94-95, 186–193 (1996).
McEwen, J.-S. et al. Catalytic reduction of NO2 with hydrogen on Pt field emitter tips: kinetic instabilities on the nanoscale. Langmuir 26, 16381–16391 (2010).
Barroo, C., De Decker, Y., Visart de Bocarmé, T. & Kruse, N. Complex oscillations patterns during the catalytic hydrogenation of NO2 over platinum nano-sized crystals. J. Phys. Chem. C. 118, 6839–6846 (2014).
Barroo, C., De Decker, Y., Visart de Bocarmé, T. & Gaspard, P. Fluctuating dynamics of nanoscale chemical oscillations: theory and experiments. J. Phys. Chem. Lett. 6, 2189–2193 (2015).
Barroo, C., De Decker, Y., Visart de Bocarmé, T. & Kruse, N. Emergence of chemical oscillations from nanosized target patterns. Phys. Rev. Lett. 117, 144501 (2016).
Segner, J., Vielhaber, W. & Ertl, G. Interaction of NO2 with a Pt(111) Surface. Isr. J. Chem. 22, 375–379 (1982).
Parker, D. H., Bartram, M. E. & Koel, B. E. Study of high coverages of atomic oxygen on the Pt(111) surface. Surf. Sci. 217, 489–510 (1989).
Barroo, C., Voorsluijs, V., Visart de Bocarmé, T., Gaspard, P. & De Decker, Y. Reconstructing stochastic attractors from nanoscale experiments on a non-equilibrium reaction. Phys. Chem. Chem. Phys. 20, 21302–21312 (2018).
Lewis, R. & Gomer, R. Adsorption of oxygen on platinum. Surf. Sci. 12, 157–176 (1968).
Dawson, P. T. & Peng, Y. K. The adsorption, desorption, and exchange reactions of oxygen, hydrogen, and water on platinum surfaces: IV. Field emission studies on the adsorption of water, hydrogen and the reaction between hydrogen and adsorbed oxygen. Surf. Sci. 92, 1–13 (1980).
Kiskinova, M., Pirug, G. & Bonzel, H. P. NO adsorption on Pt(111). Surf. Sci. 136, 285–295 (1984).
Bartram, M. E., Windham, R. G. & Koel, B. E. The molecular adsorption of nitrogen dioxide on Pt(111) studied by temperature programmed desorption and vibrational spectroscopy. Surf. Sci. 184, 57–74 (1987).
Huang, W. et al. Decomposition of NO2 on Pt(110): formation of a new oxygen adsorption state. Surf. Sci. 506, L287–L292 (2002).
Danilatos, G. D. Theory of the gaseous detector device in the environmental scanning electron microscope. Adv. Electron. Electron Phys. 78, 1–102 (1990).
Graham, M. D. et al. Effects of boundaries on pattern formation: catalytic oxidation of CO on platinum. Science 264, 80–82 (1994).
Asakura, K., Lauterbach, J., Rotermund, H. H. & Ertl, G. Spatiotemporal concentration patterns associated with the catalytic oxidation of CO and Au Covered Pt(110) Surfaces. J. Chem. Phys. 102, 8175–8184 (1995).
Heras, J. M. & Viscido, L. Work function changes upon water contamination of metal surfaces. Appl. Surf. Sci. 4, 238–241 (1980).
Karma, A. Meandering transition in two-dimensional excitable media. Phys. Rev. Lett. 65, 2824 (1990).
Suchorski, Y. et al. Visualizing catalyst heterogeneity by a multifrequential oscillating reaction. Nat. Commun. 9, 600 (2018).
Cox, M. P., Ertl, G. & Imbihl, R. Spatial self-organization of surface structure during an oscillating catalytic reaction. Phys. Rev. Lett. 54, 1725–1728 (1985).
Jakubith, S., Rotermund, H.-H., Engel, W., von Oertzen, A. & Ertl, G. Spatiotemporal concentration patterns in a surface reaction: propagating and standing waves, rotating spirals, and turbulence. Phys. Rev. Lett. 65, 3013 (1990).
Panfilov, A. & Hogeweg, P. Spiral breakup in a modified FitzHugh–Nagumo model. Phys. Lett. A 176, 295–299 (1993).
Fantauzzi, D., Mueller, J. E., Sabo, L., van Duin, A. C. T. & Jacob, T. Surface buckling and subsurface oxygen: atomistic insights into the surface oxidation of Pt(111). ChemPhysChem 16, 2797–2802 (2015).
Rotermund, H. H., Pollman, M. & Kevrekidis, I. G. Pattern formation during the CO-oxidation involving subsurface oxygen. Chaos 12, 157–163 (2002).
Banbury, J. R. A versatile ultrahigh vacuum scanning electron microscope. J. Phys. E 5, 798–802 (1972).
Acknowledgements
The authors want to acknowledge Dr Karsten Kunze for recording the EBSD map and ScopeM for the use of the equipment. C.B. thanks the Fonds de la Recherche Scientifique (F.R.S.-FNRS) and Wallonie–Bruxelles International (Excellence grant WBI.WORLD) for financial support.
Author information
Authors and Affiliations
Contributions
C.B., Z.-J.W. and M.-G.W. designed and performed the experiments and analysed the data. Z.-J.W. and M.-G.W. modified the ESEM set-up for in situ observations of gas–solid interactions. All authors contributed to the writing of the manuscript, and all authors have given approval to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3
Supplementary Video 1
An in situ SEM movie that shows the initiation of nonlinear behaviours. Hydrogen was slowly added to the NO2 flow during the experiment. The recording of the movie started at the moment where surface reactivity could be detected. The flow of H2 was fixed at this point. The movie shows propagating reaction fronts and grain-dependent dynamics, it also illustrates the presence of spillover effects that stimulate reactivity on initially non-reactive grains. The movie plays at 7 fps and shows the dynamics observed during 13 min. The images were recorded at a scanning speed of 2.86 s per frame. Experimental conditions: T = 175 °C, \(p_{{\mathrm{NO}}_2}\):\(p_{{\mathrm{H}}_{\mathrm{2}}}\) ≈ 1:10, Pto ≈ 10−2 Pa.
Supplementary Video 2
A mobile phone movie that was recorded while a small subframe of around 75 × 75 pixels was scanned at a pixel dwell time of 0.3 µs. A mobile phone was used because the ESEM does not currently allow recording of such subframes. Scanning at a reduced pixel resolution allows imaging at several hundred frames per second and thus allows us to capture relatively fast propagating wavefronts without aliasing artefacts.
Supplementary Video 3
An in situ SEM movie that shows propagating reaction fronts and meandering spiral waves. The movie plays at 7 fps and individual images were recorded at a scanning speed one frame per 0.97 s. Experimental conditions: \(p{\mathrm{NH}_2}:p{\mathrm{H}_2}\)≈ 1:8, ptot = 8.7 × 10−3 Pa, T = 192 °C.
Supplementary Video 4
Surface reactivity and propagation of reaction fronts during NO2 hydrogenation on a polycrystalline platinum foil recorded at a total pressure of 13 Pa. The movie plays at 7 fps, individual images were recorded at a scanning speed of one frame per 2.86 s.
Rights and permissions
About this article
Cite this article
Barroo, C., Wang, ZJ., Schlögl, R. et al. Imaging the dynamics of catalysed surface reactions by in situ scanning electron microscopy. Nat Catal 3, 30–39 (2020). https://doi.org/10.1038/s41929-019-0395-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0395-3
This article is cited by
-
Reverse water gas-shift reaction product driven dynamic activation of molybdenum nitride catalyst surface
Nature Communications (2024)
-
Advances in in situ/operando techniques for catalysis research: enhancing insights and discoveries
Surface Science and Technology (2024)
-
Automatic identification of crystal structures and interfaces via artificial-intelligence-based electron microscopy
npj Computational Materials (2023)
-
Periodic structural changes in Pd nanoparticles during oscillatory CO oxidation reaction
Nature Communications (2022)
-
The concept of active site in heterogeneous catalysis
Nature Reviews Chemistry (2022)