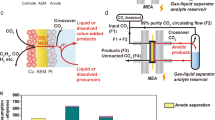
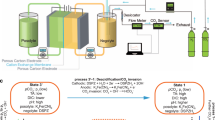
Abstract
The electrochemical conversion of carbon dioxide to value-added chemical products has been heavily explored as a promising strategy for carbon utilization. However, the direct synthesis of multi-carbon (C2+) products suffers from undesired side reactions and relatively low selectivity. Electrochemically converting CO2 to single-carbon products is much more effective and being commercially deployed. Recent studies have shown that CO can be electrochemically transformed further to C2+ at high reaction rates, high C2+ selectivity and inherently improved electrolyte stability, raising the prospect of a two-step pathway to transform CO2. In this Perspective, the progress towards high-rate CO conversion is shown alongside mechanistic insights and device designs that can improve performance even further. A techno-economic analysis of the two-step conversion process and cradle-to-gate lifecycle assessment shows the economic feasibility and improved environmental impact of a high-volume commercial process generating acetic acid and ethylene compared to the current state of the art.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
IPCC Global warming of 1.5 °C (eds. Zhai, P. et al.) (World Meteorological Organization, 2018).
Davis, S. J. et al. Net-zero emissions energy systems. Science 360, eaas9793 (2018).
Ryan, H. W. & Mark, B. 2017 Wind Technologies Market Report (US DOE, 2018).
Haegel, N. M. et al. Terawatt-scale photovoltaics: Trajectories and challenges. Science 356, 141–143 (2017).
Katelhon, A., Meys, R., Deutz, S., Suh, S. & Bardow, A. Climate change mitigation potential of carbon capture and utilization in the chemical industry. Proc. Natl Acad. Sci. USA 116, 11187–11194 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Chen, C., Khosrowabadi Kotyk, J. F. & Sheehan, S. W. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 4, 2571–2586 (2018).
Jiang, K. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 11, 893–903 (2018).
Dinh, C.-T., García de Arquer, F. P., Sinton, D. & Sargent, E. H. High rate, selective, and stable electroreduction of CO2 to CO in basic and neutral media. ACS Energy Lett. 3, 2835–2840 (2018).
Liu, Z., Yang, H., Kutz, R. & Masel, R. I. CO2 Electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371–J3377 (2018).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2017).
Li, Y. C. et al. CO2 Electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Zheng, Y. et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 141, 7646–7659 (2019).
Raciti, D. & Wang, C. Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett. 3, 1545–1556 (2018).
Zhang, H., Li, J., Cheng, M.-J. & Lu, Q. CO Electroreduction: current development and understanding of Cu-based catalysts. ACS Catal. 9, 49–65 (2018).
Jouny, M., Luc, W. & Jiao, F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 1, 748–755 (2018).
Luc, W. et al. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2, 423–430 (2019).
Ripatti, D. S., Veltman, T. R. & Kanan, M. W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 3, 240–256 (2019).
Li, J. et al. Copper adparticle enabled selective electrosynthesis of n-propanol. Nat. Commun. 9, 4614 (2018).
Zhuang, T.-T. et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal. 1, 946–951 (2018).
Pang, Y. et al. Efficient electrocatalytic conversion of carbon monoxide to propanol using fragmented copper. Nat. Catal. 2, 251–258 (2019).
Jouny, M. et al. Formation of carbon-nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Bierhals, J. Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH, 2001).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Hoang, T. T. H., Ma, S., Gold, J. I., Kenis, P. J. A. & Gewirth, A. A. Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 7, 3313–3321 (2017).
Hoang, T. T. H. et al. Nanoporous copper–silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol. J. Am. Chem. Soc. 140, 5791–5797 (2018).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Lv, J. J. et al. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 30, e1803111 (2018).
Weng, L. C., Bell, A. T. & Weber, A. Z. Towards membrane-electrode assembly systems for CO2 reduction: a modeling study. Energy Environ. Sci. 12, 1950–1968 (2019).
Li, Y. C. et al. Electrolysis of CO2 to syngas in bipolar membrane-based electrochemical cells. ACS Energy Lett. 1, 1149–1153 (2016).
Mi, Y. et al. Selective electroreduction of CO2 to C2 products over Cu3N‐derived Cu nanowires. ChemElectroChem 6, 2393–2397 (2019).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Ren, D. et al. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper(I) oxide catalysts. ACS Catal. 5, 2814–2821 (2015).
Gao, D. et al. Plasma-activated copper nanocube catalysts for efficient carbon dioxide electroreduction to hydrocarbons and alcohols. ACS Nano 11, 4825–4831 (2017).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Kim, J. et al. Branched copper oxide nanoparticles induce highly selective ethylene production by electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 141, 6986–6994 (2019).
Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl Acad. Sci. USA 114, 10560–10565 (2017).
Produce your own carbon monoxide. Haldor Topsoe www.topsoe.com/processes/carbon-monoxide/site-carbon-monoxide (accessed 11 October 2019).
Hori, Y., Takahashi, R., Yoshinami, Y. & Murata, A. Electrochemical reduction of CO at a copper electrode. J. Phys. Chem. B 101, 7075–7081 (1997).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Verdaguer-Casadevall, A. et al. Probing the active surface sites for CO reduction on oxide-derived copper electrocatalysts. J. Am. Chem. Soc. 137, 9808–9811 (2015).
Bertheussen, E. et al. Acetaldehyde as an intermediate in the electroreduction of carbon monoxide to ethanol on oxide-derived copper. Angew. Chem. Int. Ed. 55, 1450–1454 (2016).
Kortlever, R., Shen, J., Schouten, K. J., Calle-Vallejo, F. & Koper, M. T. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Cheng, T., Xiao, H. & Goddard, W. A. III. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl Acad. Sci. USA 114, 1795–1800 (2017).
Garza, A. J., Bell, A. T. & Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: pathways to C2 products. ACS Catal. 8, 1490–1499 (2018).
Lum, Y., Cheng, T., Goddard, W. A. III & Ager, J. W. Electrochemical CO reduction builds solvent water into oxygenate products. J. Am. Chem. Soc. 140, 9337–9340 (2018).
Cheng, T., Fortunelli, A. & Goddard, W. A. III. Reaction intermediates during operando electrocatalysis identified from full solvent quantum mechanics molecular dynamics. Proc. Natl Acad. Sci. USA 116, 7718–7722 (2019).
Birdja, Y. Y. & Koper, M. T. The Importance of cannizzaro-type reactions during electrocatalytic reduction of carbon dioxide. J. Am. Chem. Soc. 139, 2030–2034 (2017).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Clark, E. L. et al. Explaining the incorporation of oxygen derived from solvent water into the oxygenated products of CO reduction over Cu. J. Am. Chem. Soc. 141, 4191–4193 (2019).
Clark, E. L. & Bell, A. T. Direct observation of the local reaction environment during the electrochemical reduction of CO2. J. Am. Chem. Soc. 140, 7012–7020 (2018).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Higgins, D., Hahn, C., Xiang, C., Jaramillo, T. F. & Weber, A. Z. Gas-diffusion electrodes for carbon dioxide reduction: a new paradigm. ACS Energy Lett. 4, 317–324 (2018).
Whipple, D. T., Finke, E. C. & Kenis, P. J. Microfluidic reactor for the electrochemical reduction of carbon dioxide: the effect of pH. Electrochem. Solid State Lett. 13, B109–B111 (2010).
Dinh, C.-T., Li, Y. C. & Sargent, E. H. Boosting the single-pass conversion for renewable chemical electrosynthesis. Joule 3, 13–15 (2019).
Yang, H., Kaczur, J. J., Sajjad, S. D. & Masel, R. I. Electrochemical conversion of CO2 to formic acid utilizing Sustainion™ membranes. J. CO 2 Util. 20, 208–217 (2017).
Altarawneh, R. M. & Pickup, P. G. Product distributions and efficiencies for ethanol oxidation in a proton exchange membrane electrolysis cell. J. Electrochem. Soc. 164, F861–F865 (2017).
US Life Cycle Inventory Database (National Renewable Energy Laboratory, accessed 2 September 2019); www.lcacommons.gov/lca-collaboration/search
Schlömer S. et al. in Climate Change 2014: Mitigation of Climate Change (eds Edenhofer, O. et al.) (IPCC, Cambridge Univ. Press, 2014).
Luc, W. et al. SO2-induced selectivity change in CO2 electroreduction. J. Am. Chem. Soc. 141, 9902–9909 (2019).
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Acknowledgements
The authors acknowledge M. L. Hutchings (Yale University) for assistance in figure preparation. This material is based on work supported by the Department of Energy under award no. DE-FE0029868. The authors at the University of Delaware also thank the National Science Foundation for financial support (award no. CBET-1803200). G.S.H. acknowledges the support of the University of Delaware Blue Hen Proof of Concept programme.
Author information
Authors and Affiliations
Contributions
M.J. and G.S.H. contributed equally to this work. M.J., G.S.H. and F.J. performed data analysis and wrote the manuscript. F.J. supervised the whole project.
Corresponding authors
Ethics declarations
Competing interests
G.S.H. and F.J. are co-founders of Lectrolyst, a company developing devices for electrocatalytic conversion including carbon monoxide reduction.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1,2, Tables 1–3, Notes 1,2 and references.
Rights and permissions
About this article
Cite this article
Jouny, M., Hutchings, G.S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat Catal 2, 1062–1070 (2019). https://doi.org/10.1038/s41929-019-0388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0388-2
This article is cited by
-
Design and diagnosis of high-performance CO2-to-CO electrolyzer cells
Nature Chemical Engineering (2024)
-
An organic proton cage that is ultra-resistant to hydroxide-promoted degradation
Nature Communications (2024)
-
Well-defined diatomic catalysis for photosynthesis of C2H4 from CO2
Nature Communications (2024)
-
Tandem reactors and reactions for CO2 conversion
Nature Chemical Engineering (2024)
-
Site-selective protonation enables efficient carbon monoxide electroreduction to acetate
Nature Communications (2024)