Abstract
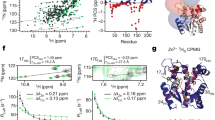
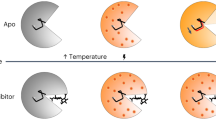
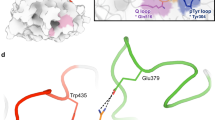
Protein conformational changes are often essential for enzyme catalysis and, in several cases, are shown to be the limiting factor for overall catalytic speed. However, a structural understanding of the corresponding transition states, needed to rationalize the kinetics, remains obscure due to their transient nature. Here, we determine the transition state ensemble of the rate-limiting conformational transition in the enzyme adenylate kinase through a synergistic approach between experimental high-pressure NMR relaxation during catalysis and molecular dynamics simulations. By comparing homologous kinases that evolved under ambient or high pressure in the deep sea, we detail transition state ensembles that differ in solvation as directly measured by the pressure dependence of catalysis. Capturing transition state ensembles begins to complete the catalytic energy landscape that is generally characterized by the structures of all intermediates and the frequencies of transitions among them.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Structure factors and refined model of piezoAdk has been deposited in the PDB under the accession code 4K46. The data that support the plots within this paper and other findings of this study are available from the corresponding author on reasonable request.
Code availability
In-house Python scripts are available from the corresponding author on request.
References
Boehr, D. D., McElheny, D., Dyson, H. J. & Wright, P. E. The dynamic energy landscape of dihydrofolate reductase catalysis. Science 313, 1638–1642 (2006).
Sekhar, A. & Kay, L. E. NMR paves the way for atomic level descriptions of sparsely populated, transiently formed biomolecular conformers. Proc. Natl Acad. Sci. USA 110, 12867–12874 (2013).
Kornev, A. P. & Taylor, S. S. Dynamics-driven allostery in protein kinases. Trends Biochem. Sci. 40, 628–647 (2015).
Baldwin, A. J. & Kay, L. E. NMR spectroscopy brings invisible protein states into focus. Nat. Chem. Biol. 5, 808–814 (2009).
Schramm, V. L. Enzymatic transition states, transition-state analogs, dynamics, thermodynamics, and lifetimes. Annu. Rev. Biochem. 80, 703–732 (2011).
Laidler, K. J. & King, M. C. The development of transition-state theory. J. Phys. Chem. 87, 2657–2664 (1983).
Royer, C. A. The nature of the transition state ensemble and the mechanisms of protein folding: a review. Arch. Biochem. Biophys. 469, 34–45 (2008).
Zhang, Y. et al. High pressure ZZ-exchange NMR reveals key features of protein folding transition states. J. Am. Chem. Soc. 138, 15260–15266 (2016).
Korzhnev, D. M. et al. Probing the transition state ensemble of a protein folding reaction by pressure-dependent NMR relaxation dispersion. J. Am. Chem. Soc. 128, 5262–5269 (2006).
Akasaka, K. Probing conformational fluctuation of proteins by pressure perturbation. Chem. Rev. 106, 1814–1835 (2006).
Roche, J. et al. Effect of internal cavities on folding rates and routes revealed by real-time pressure-jump NMR spectroscopy. J. Am. Chem. Soc. 135, 14610–14618 (2013).
Royer, C. A. Revisiting volume changes in pressure-induced protein unfolding. Biochim. Biophys. Acta 1595, 201–209 (2002).
Mitra, L., Smolin, N., Ravindra, R., Royer, C. & Winter, R. Pressure perturbation calorimetric studies of the solvation properties and the thermal unfolding of proteins in solution–experiments and theoretical interpretation. Phys. Chem. Chem. Phys. 8, 1249–1265 (2006).
Vezzi, A. et al. Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307, 1459–1461 (2005).
Kerns, S. J. et al. The energy landscape of adenylate kinase during catalysis. Nat. Struct. Mol. Biol. 22, 124–131 (2015).
Masson, P. & Balny, C. Linear and non-linear pressure dependence of enzyme catalytic parameters. Biochim. Biophys. Acta 1724, 440–450 (2005).
Hay, S. et al. Are the catalytic properties of enzymes from piezophilic organisms pressure adapted? Chembiochem 10, 2348–2353 (2009).
Hay, S., Sutcliffe, M. J. & Scrutton, N. S. Promoting motions in enzyme catalysis probed by pressure studies of kinetic isotope effects. Proc. Natl Acad. Sci. USA 104, 507–512 (2007).
Loria, J. P., Rance, M. & Palmer, A. G. III. A TROSY CPMG sequence for characterizing chemical exchange in large proteins. J. Biomol. NMR 15, 151–155 (1999).
Berry, M. B., Bae, E., Bilderback, T. R., Glaser, M. & Phillips, G. N. Jr Crystal structure of ADP/AMP complex of Escherichia coli adenylate kinase. Proteins 62, 555–556 (2006).
Muller, C. W. & Schulz, G. E. Structure of the complex between adenylate kinase from Escherichia coli and the inhibitor Ap5a refined at 1.9 a resolution—a model for a catalytic transition-state. J. Mol. Biol. 224, 159–177 (1992).
Schlitter, J., Engels, M. & Kruger, P. Targeted molecular dynamics: a new approach for searching pathways of conformational transitions. J. Mol. Graph 12, 84–89 (1994).
Dill, K. A. Dominant forces in protein folding. Biochemistry 29, 7133–7155 (1990).
Li, D., Liu, M. S. & Ji, B. Mapping the dynamics landscape of conformational transitions in enzyme: the adenylate kinase case. Biophys. J. 109, 647–660 (2015).
Arora, K. & Brooks, C. L. 3rd Large-scale allosteric conformational transitions of adenylate kinase appear to involve a population-shift mechanism. Proc. Natl Acad. Sci. USA 104, 18496–18501 (2007).
Lee, J., Joo, K., Brooks, B. R. & Lee, J. The atomistic mechanism of conformational transition of adenylate kinase investigated by Lorentzian structure-based potential. J. Chem. Theory Comput. 11, 3211–3224 (2015).
Unan, H., Yildirim, A. & Tekpinar, M. Opening mechanism of adenylate kinase can vary according to selected molecular dynamics force field. J. Comput. Aided Mol. Des. 29, 655–665 (2015).
Wang, Y., Gan, L., Wang, E. & Wang, J. Exploring the dynamic functional landscape of adenylate kinase modulated by substrates. J. Chem. Theory Comput. 9, 84–95 (2013).
Charlier, C. et al. Study of protein folding under native conditions by rapidly switching the hydrostatic pressure inside an NMR sample cell. Proc. Natl Acad. Sci. USA 115, E4169–E4178 (2018).
Kremer, W. et al. Pulsed pressure perturbations, an extra dimension in NMR spectroscopy of proteins. J. Am. Chem. Soc. 133, 13646–13651 (2011).
Hay, S., Johannissen, L. O., Hothi, P., Sutcliffe, M. J. & Scrutton, N. S. Pressure effects on enzyme-catalyzed quantum tunneling events arise from protein-specific structural and dynamic changes. J. Am. Chem. Soc. 134, 9749–9754 (2012).
Pudney, C. R. et al. Parallel pathways and free-energy landscapes for enzymatic hydride transfer probed by hydrostatic pressure. Chembiochem 10, 1379–1384 (2009).
Kalbitzer, H. R. et al. Intrinsic allosteric inhibition of signaling proteins by targeting rare interaction states detected by high-pressure NMR spectroscopy. Angew. Chem. Int. Ed. 52, 14242–14246 (2013).
Fersht, A. R., Matouschek, A. & Serrano, L. The folding of an enzyme. I. Theory of protein engineering analysis of stability and pathway of protein folding. J. Mol. Biol. 224, 771–782 (1992).
Mitra, L. et al. V i-value analysis: a pressure-based method for mapping the folding transition state ensemble of proteins. J. Am. Chem. Soc. 129, 14108–14109 (2007).
Theobald, D. L. & Wuttke, D. S. Accurate structural correlations from maximum likelihood superpositions. PLoS Comput. Biol. 4, e43 (2008).
Wolf-Watz, M. et al. Linkage between dynamics and catalysis in a thermophilic mesophilic enzyme pair. Nat. Struct. Mol. Biol. 11, 945–949 (2004).
Peterson, R. W. & Wand, A. J. Self contained high pressure cell, apparatus and procedure for the preparation of encapsulated proteins dissolved in low viscosity fluids for NMR spectroscopy. Rev. Sci. Instrum. 76, 1–7 (2005).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005).
Ahlner, A., Carlsson, M., Jonsson, B. H. & Lundstrom, P. PINT: a software for integration of peak volumes and extraction of relaxation rates. J. Biomol. NMR 56, 191–202 (2013).
Carver, J. P. & Richards, R. E. General two-site solution for chemical exchange produced dependence of T 2 upon Carr–Purcell pulse separation. J. Magn. Reson. 6, 89 (1972).
Newville, M., Stensitzki, T., Allen, D. B. & Ingargiola, A. A. LMFIT: non-linear least-square minimization and curve-fitting for Python (Zenodo, 2014); http:/lmfit.github.io/lmfit-py
Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Cryst. D 67, 271–281 (2011).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Cryst. D 67, 235–242 (2011).
Mccoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Cryst. D 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Cryst. D 66, 486–501 (2010).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
Biasini, M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 52, 255–268 (2006).
Hoover, W. G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A 31, 1695–1697 (1985).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl Phys. 52, 7182–7190 (1981).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 27–38 (1996).
Acknowledgements
We are grateful for D. V. Pachov’s preliminary TMD simulations on mesoAdk and piezoAdk and discussions, F. Pontiggia with guidance for the TMD simulations and J. Wand and R. Peterson for the initial exploratory pressure NMR experiments. Finally, we thank D. Bartlett for generously gifting P. profundom genomic DNA. This work was supported by the Howard Hughes Medical Institute, the Office of Basic Energy Sciences, the Catalysis Science Program, the US Department of Energy (grant no. DE-FG02-05ER15699 to D.K. and NIH; grant no. GM100966 to M.F.H and D.K). R.O. was supported as an HHMI fellow of the Damon Runyon Cancer Research Foundation (grant no. DRG-2114-12). Computational resources were provided by NSF XSEDE computing resources (grant no. TG-MCB090163) and the Brandeis HPCC, which is partially supported by grant no. DMR-1420382. We thank the Advanced Light Source (ALS) for beamline use. The Berkeley Center for Structural Biology is supported in part by the National Institutes of Health, National Institute of General Medical Sciences and the HHMI. The ALS is supported by the director (Office of Science, Office of Basic Energy Sciences) of the US Department of Energy under contract no. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
D.K. conceived the project idea. D.K., S.J.K, and M.F.H. developed the research plan and experimental strategy. S.J.K performed pressure-variable kinetic experiments, which included purifying the wild-type and mutant proteins, designing and assembling the high-pressure spectrophotometer, performing pressure-variable experiments and analysing the results. S.J.K, with help from R.O., also performed all NMR experiments. J.B.S, S.J.K. and R.O. performed the NMR data analyses, fitting and interpretation. Y.J.-C. purified piezoAdk for crystallization and solved the X-ray structure. M.H. performed the TMD simulations. M.H. and J.B.S analysed the TMD simulations with assistance by M.F.H. and D.K. S.J.K. and D.K. designed mutations on the basis of experimental data and TMD simulations. All authors discussed the results, which led to the overall scientific findings. J.B.S. and D.K wrote the manuscript. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Supplementary Tables 1 and 2, Supplementary Note 1 and Supplementary References
Supplementary Data 1
Triple mutant mesoAdk final structure
Supplementary Data 2
MesoAdk final structure
Supplementary Data 3
MesoAdk initial structure
Supplementary Data 4
PiezoAdk final structure
Supplementary Data 5
PiezoAdk initial structure
Supplementary Data 6
Triple mutant mesoAdk initial structure
Supplementary Video 1
Hydration of AMP-lid interface in piezoAdk
Rights and permissions
About this article
Cite this article
Stiller, J.B., Jordan Kerns, S., Hoemberger, M. et al. Probing the transition state in enzyme catalysis by high-pressure NMR dynamics. Nat Catal 2, 726–734 (2019). https://doi.org/10.1038/s41929-019-0307-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0307-6
This article is cited by
-
Structure determination of high-energy states in a dynamic protein ensemble
Nature (2022)
-
Validating the CHARMM36m protein force field with LJ-PME reveals altered hydrogen bonding dynamics under elevated pressures
Communications Chemistry (2021)
-
From structure to mechanism: skiing the energy landscape
Nature Methods (2021)
-
Proteins-Based Nanocatalysts for Energy Conversion Reactions
Topics in Current Chemistry (2020)
-
Enzyme catalysis under pressure
Nature Catalysis (2019)