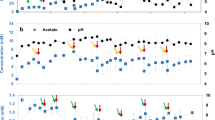
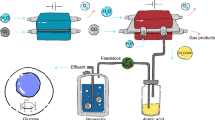
Abstract
The integration of electrochemical and microbial processes offers a unique opportunity to displace fossil carbon with CO2 and renewable energy as the primary feedstocks for carbon-based chemicals. Yet, it is unclear which strategy for CO2 activation and electron transfer to microbes has the capacity to transform the chemical industry. Here, we systematically survey experimental data for microbial growth on compounds that can be produced electrochemically, either directly or indirectly. We show that only a few strategies can support efficient electromicrobial production, where formate and methanol seem the best electron mediators in terms of energetic efficiency of feedstock bioconversion under both anaerobic and aerobic conditions. We further show that direct attachment of microbes to the cathode is highly constrained due to an inherent discrepancy between the rates of the electrochemical and biological processes. Our quantitative perspective provides a data-driven roadmap towards an economically and environmentally viable realization of electromicrobial production.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Zhu, X.-G., Long, S. P. & Ort, D. R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 19, 153–159 (2008).
Cotton, C. A. R. et al. Photosynthetic constraints on fuel from microbes. Front. Bioeng. Biotechnol. 3, 1–5 (2015).
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Claassens, N. J., Sousa, D. Z., Dos Santos, V. A. P. M., De Vos, W. M. & Van Der Oost, J. Harnessing the power of microbial autotrophy. Nat. Rev. Microbiol. 14, 692–706 (2016).
Naik, S. N., Goud, V. V., Rout, P. K. & Dalai, A. K. Production of first and second generation biofuels: a comprehensive review. Renew. Sustain. Energy Rev. 14, 578–597 (2010).
Conrado, R. J. & Gonzalez, R. Envisioning the bioconversion of methane to liquid fuels. Science 343, 621–623 (2014).
Liao, J. C., Mi, L., Pontrelli, S. & Luo, S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016).
Rabaey, K. & Rozendal, R. A. Microbial electrosynthesis—revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 8, 706–716 (2010).
Liu, C. et al. Nanowire–bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 15, 3634–3639 (2015).
Conrado, R. J., Haynes, C. A., Haendler, B. E. & Toone, E. J. in Advanced Biofuels and Bioproducts 1037–1064 (Spinger-Verlag, 2013).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Torella, J. P. et al. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc. Natl Acad. Sci. USA 112, 2337–2342 (2015).
Liu, C. et al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Khunjar, W. O., Sahin, A., West, A. C., Chandran, K. & Banta, S. Biomass production from electricity using ammonia as an electron carrier in a reverse microbial fuel cell. PLoS One 7, 1–8 (2012).
Guan, J. et al. Development of reactor configurations for an electrofuels platform utilizing genetically modified iron oxidizing bacteria for the reduction of CO2 to biochemicals. J. Biotechnol. 245, 21–27 (2017).
Li, H. et al. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335, 1596–1596 (2012).
Khan, N. E., Myers, J. A., Tuerk, A. L. & Curtis, W. R. A process economic assessment of hydrocarbon biofuels production using chemoautotrophic organisms. Bioresour. Technol. 172, 201–211 (2014).
Schuchmann, K. & Müller, V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014).
Charubin, K., Bennett, R. K., Fast, A. G. & Papoutsakis, E. T. Engineering Clostridium organisms as microbial cell-factories: challenges and opportunities. Metab. Eng. 50, 173–191 (2018).
Schiel-Bengelsdorf, B. & Dürre, P. Pathway engineering and synthetic biology using acetogens. FEBS Lett. 586, 2191–2198 (2012).
Humphreys, C. M. & Minton, N. P. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas. Curr. Opin. Biotechnol. 50, 174–181 (2018).
Köpke, M. et al. 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 77, 5467–5475 (2011).
Abubackar, H. N., Veiga, M. C. & Kennes, C. Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuel. Bioprod. Biorefin. 5, 93–114 (2011).
Knoll, A. et al. High cell density cultivation of recombinant yeasts and bacteria under non-pressurized and pressurized conditions in stirred tank bioreactors. J. Biotechnol. 132, 167–179 (2007).
Garcia-Gonzalez, L., Mozumder, M. S. I., Dubreuil, M., Volcke, E. I. P. & De Wever, H. Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catal. Today 257, 237–245 (2015).
Lyu, Z. et al. Engineering the autotroph methanococcus maripaludis for geraniol production. ACS Synth. Biol. 5, 577–581 (2016).
Nayak, D. D. & Metcalf, W. W. Cas9-mediated genome editing in the methanogenic archaeon Methanosarcina acetivorans. Proc. Natl Acad. Sci. USA 114, 2976–2981 (2017).
Anthony, C. The Biochemistry of Methylotrophs (Academic Press, 1982).
Chistoserdova, L., Kalyuzhnaya, M. G. & Lidstrom, M. E. The expanding world of methylotrophic metabolism. Annu. Rev. Microbiol. 63, 477–499 (2009).
Lawton, T. J. & Rosenzweig, A. C. Methane-oxidizing enzymes: an upstream problem in biological gas-to-liquids conversion. J. Am. Chem. Soc. 138, 9327–9340 (2016).
Cornick, N. A. & Allison, M. J. Anabolic incorporation of oxalate by Oxalobacter formigenes. Appl. Environ. Microbiol. 62, 3011–3013 (1996).
Schneider, K., Skovran, E. & Vorholt, J. A. Oxalyl-coenzyme A reduction to glyoxylate is the preferred route of oxalate assimilation in Methylobacterium extorquens AM1. J. Bacteriol. 194, 3144–3155 (2012).
Tremblay, P. L. & Zhang, T. Electrifying microbes for the production of chemicals. Front. Microbiol. 6, 201 (2015).
Sydow, A., Krieg, T., Ulber, R. & Holtmann, D. Growth medium and electrolyte—how to combine the different requirements on the reaction solution in bioelectrochemical systems using Cupriavidus necator. Eng. Life Sci. 17, 781–791 (2017).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Cui, M., Nie, H., Zhang, T., Lovley, D. & Russell, T. P. Three-dimensional hierarchical metal oxide–carbon electrode materials for highly efficient microbial electrosynthesis. Sustain. Energy Fuels 1, 1171–1176 (2017).
Aryal, N., Halder, A., Tremblay, P. L., Chi, Q. & Zhang, T. Enhanced microbial electrosynthesis with three-dimensional graphene functionalized cathodes fabricated via solvothermal synthesis. Electrochim. Acta 217, 117–122 (2016).
Jourdin, L. & Strik, D. in Functional Electrodes for Enzymatic and Microbial Electrochemical Systems 429–472 (World Scientific Publishing, 2017).
Flemming, H.-C., Wingender, J., Griebe, T. & Mayer, C. Biofilms: Recent Advances in their Study and Control (Physicochemical properties of Biofilms) (Harwood Academic, Reading, 2000).
Loferer-Kroßbacher, M., Klima, J. & Psenner, R. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis downloaded from. Appl. Environ. Microbiol. 64, 688–694 (1998).
Jourdin, L. et al. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide. Environ. Sci. Technol. 49, 13566–13574 (2015).
Buttler, A. & Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew. Sustain. Energy Rev. 82, 2440–2454 (2018).
Martín, A. J. J., Larrazábal, G. O. O. & Pérez-Ramírez, J. Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: lessons from water electrolysis. Green Chem. 17, 5114–5130 (2015).
Ma, S., Luo, R., Moniri, S., Lan, Y. & Kenis, P. J. A. Efficient electrochemical flow system with improved anode for the conversion of CO2 to CO. J. Electrochem. Soc. 161, 1124–1131 (2014).
Jeanty, P. et al. Upscaling and continuous operation of electrochemical CO2 to CO conversion in aqueous solutions on silver gas diffusion electrodes. J. CO 2 Util. 24, 454–462 (2018).
Kopljar, D., Inan, A., Vindayer, P., Wagner, N. & Klemm, E. Electrochemical reduction of CO2 to formate at high current density using gas diffusion electrodes. J. Appl. Electrochem. 44, 1107–1116 (2014).
Kopljar, D., Wagner, N. & Klemm, E. Transferring electrochemical CO2 reduction from semi-batch into continuous operation mode using gas diffusion electrodes. Chem. Eng. Technol. 39, 2042–2050 (2016).
Harnisch, F., Rosa, L. F. M., Kracke, F., Virdis, B. & Kromer, J. O. Electrifying white biotechnology: engineering and economic potential of electricity-driven bio-production. ChemSusChem 8, 758–766 (2015).
Bertuccioli, L. et al. Development of Water Electrolysis in the European Union (2014).
Commercialisation of Energy Storage in Europe: Final Report (2015); https://www.fch.europa.eu/sites/default/files/CommercializationofEnergyStorageFinal_3.pdf
Verma, S., Kim, B., Jhong, H. R. M., Ma, S. & Kenis, P. J. A. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Schmidt, O. et al. Future cost and performance of water electrolysis: an expert elicitation study. Int. J. Hydrog. Energy 42, 30470–30492 (2017).
Ainscough, C., Peterson, D. & Miller, E. Hydrogen Production Cost From PEM Electrolysis (2014); https://www.hydrogen.energy.gov/pdfs/14004_h2_production_cost_pem_electrolysis.pdf
Bazzanella, A. M. & Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemical Industry (2017); https://dechema.de/dechema_media/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry-p-20002750.pdf
Fan, Q. et al. Electrochemical CO2 reduction to C2 + species: heterogeneous electrocatalysts, reaction pathways and optimization strategies. Mater. Today Energy 10, 280–301 (2018).
Gotz, M. et al. Renewable power-to-gas: a technological and economic review. Renew. Energy 85, 1371–1390 (2016).
Bailera, M., Lisbona, P., Romeo, L. M. & Espatolero, S. Power to gas projects review: lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 69, 292–312 (2017).
Mignard, D. & Pritchard, C. Processes for the synthesis of liquid fuels from CO2 and marine energy. Chem. Eng. Res. Des. 84, 828–836 (2006).
Szima, S. & Cormos, C. C. Improving methanol synthesis from carbon-free H2 and captured CO2: a techno-economic and environmental evaluation. J. CO 2 Util. 24, 555–563 (2018).
Kezibri, N. & Bouallou, C. Conceptual design and modelling of an industrial scale power to gas-oxycombustion power plant. Int. J. Hydrog. Energy 42, 19411–19419 (2017).
Gruber, M. et al. Power-to-gas through thermal integration of high-temperature steam electrolysis and carbon dioxide methanation—experimental results. Fuel Process. Technol. J. 181, 61–74 (2018).
Salomone, F., Giglio, E., Ferrero, D., Santarelli, M. & Pirone, R. Techno-economic modelling of a power-to-gas system based on SOEC electrolysis and CO2 methanation in a RES-based electric grid. Chem. Eng. J. https://doi.org/10.1016/j.cej.2018.10.170 (2018).
Bulushev, D. A. & Ross, J. R. H. Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates. Catal. Rev. 60, 566–593 (2018).
Alvarez, A. et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 117, 9804–9838 (2017).
Wang, L. et al. Greening ammonia toward the solar ammonia refinery. Joule 2, 1–20 (2018).
Martin, A. J., Shinagawa, T. & Perez-Ramırez, J. Electrocatalytic reduction of nitrogen: from Haber–Bosch to ammonia artificial leaf. Chem 5, 1–21 (2019).
Kalz, K. F. et al. Future challenges in heterogeneous catalysis: understanding catalysts under dynamic reaction conditions. ChemCatChem 9, 17–29 (2017).
Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 15, 4399–4981 (2015).
Weusthuis, R. A., Lamot, I., van der Oost, J. & Sanders, J. P. M. C. Microbial production of bulk chemicals: development of anaerobic processes. Trends Biotechnol. 29, 153–158 (2011).
García-Ochoa, F. & Gómez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol. Adv. 27, 153–176 (2009).
Greenblatt, J. B., Miller, D. J., Ager, J. W., Houle, F. A. & Sharp, I. D. The technical and energetic challenges of separating (photo)electrochemical carbon dioxide reduction products. Joule 2, 381–420 (2018).
Yang, H., Kaczur, J. J., Sajjad, S. D. & Masel, R. I. CO2 conversion to formic acid in a three compartment cell with SustainionTM membranes. ECS Trans. 77, 1425–1431 (2017).
Pfeifenschneider, J., Brautaset, T. & Wendisch, V. F. Methanol as carbon substrate in the bio-economy: metabolic engineering of aerobic methylotrophic bacteria for production of value-added chemicals. Biofuel. Bioprod. Biorefin. 11, 719–731 (2017).
Tremblay, P.-L., Höglund, D., Koza, A., Bonde, I. & Zhang, T. Adaptation of the autotrophic acetogen Sporomusa ovata to methanol accelerates the conversion of CO2 to organic products. Sci. Rep. 5, 16168 (2015).
Nicholls, P. Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 67, 610–616 (1975).
Warnecke, T. & Gill, R. T. Organic acid toxicity, tolerance and production in Escherichia coli biorefining applications. Microb. Cell Fact. 4, 25 (2005).
Kim, P., Kim, J.-H. & Oh, D.-K. Improvement in cell yield of Methylobacterium sp. by reducing the inhibition of medium components for poly-β-hydroxybutyrate production. World J. Microbiol. Biotechnol. 19, 357–361 (2003).
Choi, J.-H., Kim, J. H., Daniel, M. & Lebeault, J. M. Optimization of growth medium and poly-hydroxybutyric acid production from methanol in Methylobacterium organophilum. Microbiol. Biotechnol. Lett. 17, 392–396 (1989).
Ahn, J. H., Bang, J., Kim, W. J. & Lee, S. Y. Formic acid as a secondary substrate for succinic acid production by metabolically engineered Mannheimia succiniciproducens. Biotechnol. Bioeng. 114, 2837–2847 (2017).
Grunwald, S. et al. Kinetic and stoichiometric characterization of organoautotrophic growth of Ralstonia eutropha on formic acid in fed-batch and continuous cultures. Microb. Biotechnol. 8, 155–163 (2015).
Diender, M., Stams, A. J. M. & Sousa, D. Z. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 6, 1275 (2015).
Bar-Even, A., Noor, E., Flamholz, A. & Milo, R. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes. Biochim. Biophys. Acta 1827, 1039–1047 (2013).
Bar-Even, A. Formate assimilation: the metabolic architecture of natural and synthetic pathways. Biochemistry 55, 3851–3863 (2016).
Yishai, O., Bouzon, M., Döring, V. & Bar-Even, A. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli. ACS Synth. Biol. 7, 2023–2028 (2018).
Schink, B. & Friedrich, M. Phosphite oxidation by sulphate reduction. Nature 406, 37 (2000).
Lehtinen, T. et al. Production of long chain alkyl esters from carbon dioxide and electricity by a two-stage bacterial process. Bioresour. Technol. 243, 30–36 (2017).
Hu, P. et al. Integrated bioprocess for conversion of gaseous substrates to liquids. Proc. Natl Acad. Sci. USA 113, 14–19 (2016).
Al Rowaihi, I. S. et al. A two-stage biological gas to liquid transfer process to convert carbon dioxide into bioplastic. Bioresour. Technol. Rep. 1, 61–68 (2018).
Cordier, J. L., Butsch, B. M., Birou, B. & von Stockar, U. The relationship between elemental composition and heat of combustion of microbial biomass. Appl. Microbiol. Biotechnol. 25, 305–312 (1987).
Pikaar, I. et al. Carbon emission avoidance and capture by producing in-reactor microbial biomass based food, feed and slow release fertilizer: potentials and limitations. Sci. Total Environ. 644, 1525–1530 (2018).
Spurgeon, J. M. & Kumar, B. A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci. 11, 1536–1551 (2018).
Li, X. et al. Greenhouse gas emissions, energy efficiency and cost of synthetic fuel production using electrochemical CO2 conversion and the Fischer–Tropsch process. Energy Fuels 30, 5980–5989 (2016).
Delacourt, C., Ridgway, P. L., Kerr, J. B. & Newman, J. Design of an electrochemical cell making syngas (CO + H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 155, B42–B49 (2008).
Bard, A., Parsons, R. & Jordan, J. Standard Potentials in Aqueous Solution (CRC Press, Boca Raton, 1985).
Del Castillo, A. et al. Sn nanoparticles on gas diffusion electrodes: synthesis, characterization and use for continuous CO2 electroreduction to formate. J. CO 2 Util. 18, 222–228 (2017).
Schuster, T. & Rüdt von Collenberg, L. Investitionsrechnung: Kapitalwert, Zinsfuß, Annuität, Amortisation (Springer Gabler, Wiesbaden, 2017).
Quarterly report on European Electricity Markets (European Commission, 2018); https://ec.europa.eu/energy/sites/ener/files/documents/quarterly_report_on_european_electricity_markets_q2_2018.pdf
Posdziech, O. & Schwarze, K. Efficient hydrogen production for industry and electricity storage via high-temperature electrolysis. Int. J. Hydrog. Energy https://doi.org/10.1016/j.ijhydene.2018.05.169 (2018).
Ebbesen, S. D., Jensen, S. H., Hauch, A. & Mogensen, M. B. High temperature electrolysis in alkaline cells, solid proton conducting cells and solid oxide cells. Chem. Rev. 114, 10697–10734 (2014).
Patyk, A., Bachmann, T. M. & Brisse, A. Life cycle assessment of H2 generation with high temperature electrolysis. Int. J. Hydrog. Energy 38, 3865–3880 (2013).
Acknowledgements
The authors thank R. Milo, S. Geiger, A. Gago, A. Flamholz, H. He, D. Holtmann, F. Kensy, E. Noor and A. Satanowski for helpful discussions and critical reading of the manuscript. This work received funding from the Max Planck Society and from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 763911 (Project eForFuel). N.J.C. is supported by The Netherlands Organization for Scientific Research (NWO) through a Rubicon Grant (project 019.163LW.035).
Author information
Authors and Affiliations
Contributions
A.B.-E. conceptualized and supervised the research. N.J.C., C.A.R.C. and A.B.-E. designed and performed the quantitative microbial analysis. D.K. performed the electrolysis cost analysis. N.J.C., C.A.R.C., D.K. and A.B.-E. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A.B.-E. is cofounder of b.fab, exploring the commercialization of microbial bioproduction using formate as feedstock. The company was not involved in any way in performing or funding this study.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1, Supplementary Table 1, Supplementary references
Supplementary Data 1
Growth parameters collected from literature and calculated energetic efficiencies and electron consumption rates.
Rights and permissions
About this article
Cite this article
Claassens, N.J., Cotton, C.A.R., Kopljar, D. et al. Making quantitative sense of electromicrobial production. Nat Catal 2, 437–447 (2019). https://doi.org/10.1038/s41929-019-0272-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-019-0272-0
This article is cited by
-
Engineering osmolysis susceptibility in Cupriavidus necator and Escherichia coli for recovery of intracellular products
Microbial Cell Factories (2023)
-
Enhancement of acetate production in hydrogen-mediated microbial electrosynthesis reactors by addition of silica nanoparticles
Bioresources and Bioprocessing (2023)
-
Synergistic investigation of natural and synthetic C1-trophic microorganisms to foster a circular carbon economy
Nature Communications (2023)
-
Construction and modular implementation of the THETA cycle for synthetic CO2 fixation
Nature Catalysis (2023)
-
Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering
Nature Catalysis (2022)