Abstract
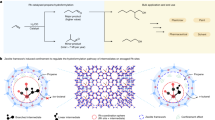
Electrochemical CO2 reduction to formate provides an avenue to reduce globally accelerating CO2 emissions and produce value-added products. Unfortunately, high selectivity in formate electrosynthesis has thus far only been achieved at highly cathodic potentials. Here we use density functional theory to investigate the effect of alloying Cu and Sn on the activity and selectivity towards formate. A theoretical thermodynamic analysis of the reaction energetics suggests that the incorporation of copper into tin could suppress hydrogen evolution and CO production, thus favouring formate generation. Consistent with theoretical trends, the designed CuSn3 catalysts by co-electrodeposition exhibit a Faradaic efficiency of 95% towards formate generation at −0.5 V versus RHE. Furthermore, the catalysts show no degradation over 50 h of operation. In situ Sn L3-edge and Cu K-edge X-ray absorption spectroscopy indicate electron donation from Sn to Cu, which indicates positive oxidation states of Sn in CuSn3 under operating conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information and Supplementary Data. Extra data are available from the corresponding author upon reasonable request.
Change history
05 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41929-021-00619-9
References
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1215 (2015).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Zheng, X. et al. Theory-driven design of high-valence metal sites for water oxidation confirmed using in situ soft X-ray absorption. Nat. Chem. 10, 149–154 (2017).
Jhong, H. R., Ma, S. & Kenis, P. J. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges and future opportunities. Nanotechnology 2, 191–199 (2013).
Feaster, J., Shi, C., Cave, E. R., Nørskov, J. & Jaramillo, T. F. Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. ACS Catal. 7, 4822–4827 (2017).
Zheng, X., De Luna, P., de Arquer, F. P., Yang, P. & Sargent, E. H. Sulfur-modulated tin sites enable highly selective electrochemical reduction of CO2 to formate. Joule 1, 1–12 (2017).
Du, D., Lan, R., Humphreys, J. & Tao Progress in inorganic cathode catalysts for electrochemical conversion of carbon dioxide into formate or formic acid. J. Appl. Electrochem. 47, 661–678 (2017).
Reda, T., Plugge, C. M., Abram, N. J. & Hirst, J. Reversible interconversion of carbon dioxide and formate by an electroactive enzyme. Proc. Natl Acad. Sci. USA 105, 10654–10658 (2008).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energ. Environ. Sci. 5, 7050–7060 (2012).
Chen, Y. & Kanan, M. W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts. J. Am. Chem. Soc. 134, 1986–1989 (2012).
Zhu, Q. et al. Efficient reduction of CO2 into formic acid on a lead or tin electrode using an ionic liquid catholyte mixture. Angew. Chem. Int. Ed. 55, 9012–9016 (2016).
Li, F., Chen, L., Knowles, G. P., MacFarlane, D. R. & Zhang, J. Hierarchical mesoporous SnO2 nanosheets on carbon cloth: a robust and flexible electrocatalyst for CO2 reduction. Angew. Chem. Int. Ed. 56, 505–509 (2017).
Lee, C. H. & Kanan, M. W. Controlling H+ vs CO2 reduction selectivity on Pb electrodes. ACS Catal. 5, 465–469 (2015).
Min, X. & Kanan, M. W. Pd-catalyzed electrohydrogenation of carbon dioxide to formate: high mass activity at low overpotential and identification of the deactivation pathway. J. Am. Chem. Soc. 137, 4701–4708 (2015).
Zhang, S., Kang, P. & Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 136, 1734–1737 (2014).
Lei, F. et al. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 7, 12697 (2016).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Sun, K., Cheng, T., Wu, L. Z., Goddard, W. A. & Wang, Z. Ultrahigh mass activity for carbon dioxide reduction enabled by gold–iron core–shell nanoparticles. J. Am. Chem. Soc. 139, 15608–15611 (2017).
Kortlever, R., Peters, I., Koper, S. & Koper, M. T. Electrochemical CO2 reduction to formic acid at low overpotential and with high Faradaic efficiency on carbon-supported bimetallic Pd–Pt nanoparticles. ACS Catal. 5, 3916–3923 (2015).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 11002–11014 (2013).
Villars, P. & Calvert, L. D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases Vol. 4, 5366 (ASM International, Materials Park, Russell Township, 1991).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energ. Environ. Sci. 3, 1311–1315 (2010).
Peterson, A. A. & Nørskov, J. K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 3, 251–258 (2012).
Yoo, J. S., Christensen, R., Vegge, T., Nørskov, J. & Studt, F. Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid. ChemSusChem 9, 358–363 (2015).
Medford, A. et al. Assessing the reliability of calculated catalytic ammonia synthesis rates. Science 345, 197–202 (2014).
Bicelli, L. P., Bozzini, B., Mele, C. & D’Urzo, L. A review of nanostructural aspects of metal electrodeposition. Int. J. Electron. Sci. 3, 356–408 (2008).
Proffen, K., Page, K., Seshadri, R. & Cheetham A pair distribution function for nanoparticle studies. Los Alamos Sci. 30, 161–164 (2006).
Liu, Z., Handa, K., Kaibuchi, K., Tanaka, Y. & Kawai, J. Comparison of the Sn L edge X-ray absorption spectra and the corresponding electronic structure in Sn, SnO and SnO2. J. Electron. Spectros. Relat. Phenomena 135, 155–158 (2004).
Gorlin, Y. et al. In situ X‑ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction. J. Am. Chem. Soc. 135, 8525–8534 (2013).
Henkelman, G., Arnaldsson, A. & Jonsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Wang, D. et al. Atomic layer deposited coatings to significantly stabilize anodes for Li ion batteries: effects of coating thickness and the size of anode particles. J. Mater. Chem. A 2, 2306–2312 (2014).
Horsley, J. A. Relationship between the area of L2,3 X-ray absorption edge resonances and the d orbital occupancy in compounds of platinum and iridium. J. Chem. Phys. 76, 1451–1460 (1982).
Cramer, S. P., Eccles, T. K., Kutzler, F. W. & Hodgson, K. Molybdenum X-ray absorption edge spectra. The chemical state of molybdenum in nitrogenase. J. Am. Chem. Soc. 98, 1287–1289 (1975).
Sun, S., Li, H. & Xu, Z. J. Impact of surface area in evaluation of catalyst activity. Joule 2, 1024–1027 (2018).
Giannozzi, P. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 6, 8245–8257 (1994).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7896 (1990).
Bahn, S. & Jacobsen, K. An object-oriented scripting interface to a legacy electronic structurecode. Comput. Sci. Eng. 2, 56–67 (2002).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Liu, X. et al. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 8, 15438 (2017).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Nørskov, J., Rossmeisl, J., Logadottir, A. & Lindqvist, L. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 59, 12301–12305 (1999).
Li, C. W., Ciston, J. & Kanan, M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014).
Bai, X. et al. Exclusive formation of formic acid from CO2 electroreduction by a tunable Pd–Sn alloy. Angew. Chem. Int. Ed. 56, 12219–12223 (2017).
Acknowledgements
This work was supported by the Department of Energy (DOE), Office of Basic Energy Sciences, Division of Materials Sciences and Engineering (contract no. DE-AC02-76SF00515). Theoretical calculations were based on work performed by the Joint Center for Artificial Photosynthesis, a DOE Energy Innovation Hub, supported through the Office of Science of the US Department of Energy under award no. DE-SC0004993. Theoretical calculations used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US Department of Energy under contract no. DE-AC02- 05CH11231. The authors thank S. Fakra and acknowledge use of Beamline 10.3.2 at the Advanced Light Source for collection of XAS data. The Advanced Light Source and Molecular Foundry are supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231. The authors thank R. Davis and E. Jonathan from SSRL for XAS measurements. Use of the Stanford Synchrotron Radiation Lightsource (SLAC National Accelerator Laboratory) is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. The authors acknowledge support with electron microscopy from the Stanford Nano Shared Facilities. Y.J. thanks the Knut & Alice Wallenberg Foundation for financial support through the ‘Wallenberg Postdoctoral Scholarship Program’ at Stanford.
Author information
Authors and Affiliations
Contributions
X.Z. and Y.C. conceived the research and designed the experiments. X.Z., J.T., J.W. and B.L. performed electrochemical measurements. Y.J. and K.C. carried out simulation parts. M.F.T. supervised and designed X-ray absorption and XRD experiments. X.Z., K.L. and H.-G.S. performed the X-ray absorption measurements. H.-G.S. carried out XRD measurements. Y.L. performed TEM measurements. All authors discussed the results and assisted during manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–19, Supplementary Tables 1–3 and Supplementary References
Supplementary Data
Optimized Cartesian coordinates of the CuSn and CuSn3 models
About this article
Cite this article
Zheng, X., Ji, Y., Tang, J. et al. RETRACTED ARTICLE: Theory-guided Sn/Cu alloying for efficient CO2 electroreduction at low overpotentials. Nat Catal 2, 55–61 (2019). https://doi.org/10.1038/s41929-018-0200-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0200-8
This article is cited by
-
Rational design of bimetallic catalysts for electrochemical CO2 reduction reaction: A review
Science China Chemistry (2023)
-
Atomic design of carbon-based dual-metal site catalysts for energy applications
Nano Research (2023)
-
Research progress on electrochemical CO2 reduction for Cu-based single-atom catalysts
Science China Materials (2023)
-
Highly selective electrochemical CO2 reduction to formate using Sn@Cu electrocatalyst
Journal of Applied Electrochemistry (2023)
-
Enhancing the electrochemical performance of ZnO anode by novel additive of MoS2–SnO2 nanocomposite for the zinc alkaline battery application
Journal of Materials Science: Materials in Electronics (2022)