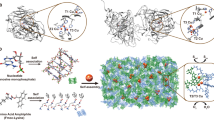
Abstract
Artificial metalloenzymes that contain protein-anchored synthetic catalysts are attracting increasing interest. An exciting, but still unrealized advantage of non-covalent anchoring is its potential for reversibility and thus component recycling. Here we present a siderophore–protein combination that enables strong but redox-reversible catalyst anchoring, as exemplified by an artificial transfer hydrogenase (ATHase). By linking the iron(iii)-binding siderophore azotochelin to an iridium-containing imine-reduction catalyst that produces racemic product in the absence of the protein CeuE, but a reproducible enantiomeric excess if protein bound, the assembly and reductively triggered disassembly of the ATHase was achieved. The crystal structure of the ATHase identified the residues involved in high-affinity binding and enantioselectivity. While in the presence of iron(iii), the azotochelin-based anchor binds CeuE with high affinity, and the reduction of the coordinated iron(iii) to iron(ii) triggers its dissociation from the protein. Thus, the assembly of the artificial enzyme can be controlled via the iron oxidation state.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoarau, M., Hureau, C., Gras, E. & Faller, P. Coordination complexes and biomolecules: a wise wedding for catalysis upgrade. Coord. Chem. Rev. 308, 445–459 (2016).
Yu, F. et al. Protein design: toward functional metalloenzymes. Chem. Rev. 114, 3495–3578 (2014).
Lu, Y., Yeung, N., Sieracki, N. & Marshall, N. M. Design of functional metalloproteins. Nature 460, 855–862 (2009).
Lewis, J. C. Artificial metalloenzymes and metallopeptide catalysts for organic synthesis. ACS Catal. 3, 2954–2975 (2013).
Bos, J. & Roelfes, G. Artificial metalloenzymes for enantioselective catalysis. Curr. Opin. Chem. Biol. 19, 135–143 (2014).
Matsuo, T. & Hirota, S. Artificial enzymes with protein scaffolds: structural design and modification. Biorg. Med. Chem. 22, 5638–5656 (2014).
Pàmies, O., Diéguez, M. & Bäckvall, J.-E. Artificial metalloenzymes in asymmetric catalysis: key developments and future directions. Adv. Synth. Catal. 357, 1567–1586 (2015).
Heinisch, T. & Ward, T. R. Artificial metalloenzymes based on the biotin–streptavidin technology: challenges and opportunities. Acc. Chem. Res. 49, 1711–1721 (2016).
Sauer, D. F. et al. A highly active biohybrid catalyst for olefin metathesis in water: impact of a hydrophobic cavity in a β-barrel protein. ACS Catal. 5, 7519–7522 (2015).
Cotelle, Y., Lebrun, V., Sakai, N., Ward, T. R. & Matile, S. Anion-π enzymes. ACS Cent. Sci. 2, 388–393 (2016).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Jeschek, M., Panke, S. & Ward, T. R. Artificial metalloenzymes on the verge of new-to-nature metabolism. Trends Biotechnol. 36, 60–72 (2018).
Wilson, M. E. & Whitesides, G. M. Conversion of a protein to a homogeneous asymmetric hydrogenation catalyst by site-specific modification with a diphosphinerhodium(i) moiety. J. Am. Chem. Soc. 100, 306–307 (1978).
Jeschek, M. et al. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 537, 661–665 (2016).
Hyster, T. K., Knörr, L., Ward, T. R. & Rovis, T. Biotinylated Rh(iii) complexes in engineered streptavidin for accelerated asymmetric C–H activation. Science 338, 500–503 (2012).
Liu, Z. et al. Upregulation of an artificial zymogen by proteolysis. Angew. Chem. Int. Ed. 55, 11587–11590 (2016).
Monnard, F. W., Nogueira, E. S., Heinisch, T., Schirmer, T. & Ward, T. R. Human carbonic anhydrase II as host protein for the creation of artificial metalloenzymes: the asymmetric transfer hydrogenation of imines. Chem. Sci. 4, 3269–3274 (2013).
Matsuo, T. et al. Creation of an artificial metalloprotein with a Hoveyda–Grubbs catalyst moiety through the intrinsic inhibition mechanism of α-chymotrypsin. Chem. Commun. 48, 1662–1664 (2012).
Key, H. M., Dydio, P., Clark, D. S. & Hartwig, J. F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016).
Ying, L.-Q. & Branchaud, B. P. Design of a reversible biotin analog and applications in protein labeling, detection, and isolation. Chem. Commun. 47, 8593–8595 (2011).
Terai, T. et al. Artificial ligands of streptavidin (ALiS): discovery, characterization, and application for reversible control of intracellular protein transport. J. Am. Chem. Soc. 137, 10464–10467 (2015).
Pollheimer, P. et al. Reversible biofunctionalization of surfaces with a switchable mutant of avidin. Bioconjug. Chem. 24, 1656–1668 (2013).
Dwyer, M. A. & Hellinga, H. W. Periplasmic binding proteins: a versatile superfamily for protein engineering. Curr. Opin. Struct. Biol. 14, 495–504 (2004).
Miethke, M. & Marahiel, M. A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 71, 413–451 (2007).
Hider, R. C. & Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 27, 637–657 (2010).
Schalk, I. J. & Guillon, L. Fate of ferrisiderophores after import across bacterial outer membranes: different iron release strategies are observed in the cytoplasm or periplasm depending on the siderophore pathways. Amino Acids 44, 1267–1277 (2013).
Wilson, B. R., Bogdan, A. R., Miyazawa, M., Hashimoto, K. & Tsuji, Y. Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol. Med. 22, 1077–1090 (2016).
Ganne, G. et al. Iron release from the siderophore pyoverdine in Pseudomonas aeruginosa involves three new actors: FpvC, FpvG, and FpvH. ACS Chem. Biol. 12, 1056–1065 (2017).
Raines, D. J. et al. Bacteria in an intense competition for iron: key component of the Campylobacter jejuni iron uptake system scavenges enterobactin hydrolysis product. Proc. Natl Acad. Sci. USA 113, 5850–5855 (2016).
Raines, D. J., Moroz, O. V., Wilson, K. S. & Duhme-Klair, A.-K. Interactions of a periplasmic binding protein with a tetradentate siderophore mimic. Angew. Chem. Int. Ed. 52, 4595–4598 (2013).
Zimbron, J. M. et al. A dual anchoring strategy for the localization and activation of artificial metalloenzymes based on the biotin–streptavidin technology. J. Am. Chem. Soc. 135, 5384–5388 (2013).
Dürrenberger, M. et al. Artificial transfer hydrogenases for the enantioselective reduction of cyclic imines. Angew. Chem. Int. Ed. 50, 3026–3029 (2011).
Madern, N., Talbi, B. & Salmain, M. Aqueous phase transfer hydrogenation of aryl ketones catalysed by achiral ruthenium(ii) and rhodium(iii) complexes and their papain conjugates. Appl. Organomet. Chem. 27, 6–12 (2013).
Chimiak, A. & Neilands, J. B. Lysine analogues of siderophores. Struct. Bond. 58, 89–96 (1984).
Lo, C., Ringenberg, M. R., Gnandt, D., Wilson, Y. & Ward, T. R. Artificial metalloenzymes for olefin metathesis based on the biotin-(strept)avidin technology. Chem. Commun. 47, 12065–12067 (2011).
Stookey, L. L. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42, 779–781 (1970).
Ruff, A., Kirby, C., Chan, B. C. & O’Connor, A. R. Base-free transfer hydrogenation of ketones using Cp*Ir(pyridinesulfonamide)Cl precatalysts. Organometallics 35, 327–335 (2016).
Townsend, T. M., Kirby, C., Ruff, A. & O’Connor, A. R. Transfer hydrogenation of aromatic and linear aldehydes catalyzed using Cp*Ir(pyridinesulfonamide)Cl complexes under base-free conditions. J. Organomet. Chem. 843, 7–13 (2017).
Fu, Y. et al. The contrasting catalytic efficiency and cancer cell antiproliferative activity of stereoselective organoruthenium transfer hydrogenation catalysts. Dalton Trans. 45, 8367–8378 (2016).
Soni, R. et al. The importance of the N–H bond in Ru/TsDPEN complexes for asymmetric transfer hydrogenation of ketones and imines. Org. Biomol. Chem. 9, 3290–3294 (2011).
Václavík, J. et al. Experimental and theoretical perspectives of the Noyori–Ikariya asymmetric transfer hydrogenation of imines. Molecules 19, 6987–7007 (2014).
Müller, A., Wilkinson, A. J., Wilson, K. S. & Duhme-Klair, A.-K. An [{Fe(mecam)}2]6− bridge in the crystal structure of a ferric enterobactin binding protein. Angew. Chem. Int. Ed. 45, 5132–5136 (2006).
Wilde, E. J. et al. Interactions of the periplasmic binding protein CeuE with Fe(iii) n-LICAM4− siderophore analogues of varied linker length. Sci. Rep. 7, 45941 (2017).
Scarrow, R. C., Ecker, D. J., Ng, C., Liu, S. & Raymond, K. N. Iron(iii) coordination chemistry of linear dihydroxyserine compounds derived from enterobactin. Inorg. Chem. 30, 900–906 (1991).
Okamoto, Y., Köhler, V. & Ward, T. R. An NAD(P)H-dependent artificial transfer hydrogenase for multienzymatic cascades. J. Am. Chem. Soc. 138, 5781–5784 (2016).
Chimiak, A. & Neilands, J. B. Lysine analogues of siderophores. Struct. Bond. 58, 89–96 (1984).
White, C., Yates, A., Maitlis, P. M. & Heinekey, D. M. (η5‐Pentamethylcyclopentadienyl) rhodium and-iridium compounds. Inorg. Synth. 29, 228–234 (2007).
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC, grant EP/L024829/1) and Biotechnology and Biological Sciences Research Council (BBSRC) for financial support, the Diamond Light Source for access to beamlines I03 and I04 (proposal; mx-13587) and J. Turkenburg and S. Hart for assistance with data collection. In addition, experimental support by K. Heaton (mass spectrometry), A. Leech (CD spectroscopy and mass spectrometry), A. Dixon (HPLC), J. H. Peng (catalyst preparation) and A. C. Whitwood (small-molecule crystallography) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
The experiments were performed by D.J.R. (chemical synthesis, development of catalysts and screening protocols, binding constant determination, redox reversibility tests), J.E.C. (artificial enzyme production and characterization, catalytic screening, redox reversibility tests), A.-K.D.-K. (redox reversibility tests), E.V.B. (protein production and protein crystal growth), E.J.D (protein crystal structure refinement) and K.S.W. (protein crystal structure refinement). K.S.W. (structural biology) and A.-K.D.-K. (chemistry) supervised and directed the project. The manuscript was produced by D.J.R and A.-K.D.-K. based on contributions by all authors. All authors have given approval to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Tables 1-3, Supplementary Figures 1–10, Supplementary References
Rights and permissions
About this article
Cite this article
Raines, D.J., Clarke, J.E., Blagova, E.V. et al. Redox-switchable siderophore anchor enables reversible artificial metalloenzyme assembly. Nat Catal 1, 680–688 (2018). https://doi.org/10.1038/s41929-018-0124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0124-3
This article is cited by
-
Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression
Nature Communications (2022)
-
Engineering new catalytic activities in enzymes
Nature Catalysis (2020)
-
An artificial metalloenzyme biosensor can detect ethylene gas in fruits and Arabidopsis leaves
Nature Communications (2019)
-
Biocompatibility and therapeutic potential of glycosylated albumin artificial metalloenzymes
Nature Catalysis (2019)
-
Reversible catalyst anchoring
Nature Catalysis (2018)