Abstract
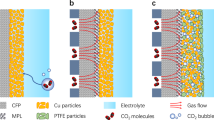
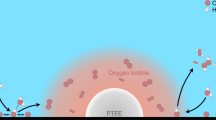
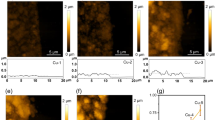
Electrochemical CO2 reduction is a critical approach to reducing the globally accelerating CO2 emission and generating value-added products. Despite great efforts to optimize catalyst activity and selectivity, facilitating the catalyst accessibility to high CO2 concentrations while maintaining electrode durability remains a significant challenge. Here, we designed a catalytic system that mimics the alveolus structure in mammalian lungs with high gas permeability but very low water diffusibility, enabling an array of three-phase catalytic interfaces. Flexible, hydrophobic, nanoporous polyethylene membranes with high gas permeability were used to enable efficient CO2 access and a high local alkalinity on the catalyst surface at different CO2 flow rates. Such an alveolus-mimicking structure generates a high CO production Faradaic efficiency of 92% and excellent geometric current densities of CO production (25.5 mA cm−2) at −0.6 V versus the reversible hydrogen electrode, with a very thin catalyst thickness of 20−80 nm.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McDonald, T. M. et al. Cooperative insertion of CO2 in diamine-appended metal–organic frameworks. Nature 519, 303–308 (2015).
Liu, C., Colón, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016).
Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016).
Cabán-Acevedo, M. et al. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 14, 1245–1251 (2015).
Liu, C. et al. Nanowire–bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals. Nano Lett. 15, 3634–3639 (2015).
Feng, X., Jiang, K., Fan, S. & Kanan, M. W. Grain-boundary-dependent CO2 electroreduction activity. J. Am. Chem. Soc. 137, 4606–4609 (2015).
Sen, S., Liu, D. & Palmore, G. T. R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 4, 3091–3095 (2014).
Lu, Q. et al. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 5, 3242 (2014).
Jiang, K. et al. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Sreekanth, N. & Phanim, K. L. Selective reduction of CO2 to formate through bicarbonate reduction on metal electrodes: new insights gained from SG/TC mode of SECM. Chem. Commun. 50, 11143–11146 (2014).
Asadi, M. et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 5, 4470 (2014).
Wu, J. et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 9, 5364–5371 (2015).
Wu, B. & Zheng, N. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8, 168–197 (2013).
Raciti, D., Livi, K. J. & Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 15, 6829–6835 (2015).
Zhu, W. et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 135, 16833–16836 (2013).
Hall, A. S., Yoon, Y., Wuttig, A. & Surendranath, Y. Mesostructure-induced selectivity in CO2 reduction catalysis. J. Am. Chem. Soc. 137, 14834–14837 (2015).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Mettela, G. & Kulkarni, G. U. Facet selective etching of Au microcrystallites. Nano Res. 8, 2925–2934 (2015).
Kim, D., Resasco, J., Yu, Y., Asiri, A. M. & Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 5, 4948 (2014).
Wang, H., Chen, Y., Hou, X., Ma, C. & Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 18, 3250–3256 (2016).
Du, L. et al. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 29, 314–322 (2016).
Verma, S., Lu, X., Ma, S., Masel, R. & Kenis, P. J. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 18, 7075–7084 (2016).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Gauthier, E., Duan, Q., Hellstern, T. & Benziger, J. Water flow in, through, and around the gas diffusion layer. Fuel Cells 12, 835–847 (2012).
Yu, S. et al. Study on hydrophobicity degradation of gas diffusion layer in proton exchange membrane fuel cells. Energy Convers. Manag. 76, 301–306 (2013).
Wagner, K., Tiwari, P., Swiegers, G. & Wallace, G. An electrochemical cell with Gortex-based electrodes capable of extracting pure hydrogen from highly dilute hydrogen–methane mixtures. Energy Environ. Sci. 11, 172–184 (2018).
Verma, S. et al. Insights into the low overpotential electroreduction of CO2 to CO on a supported gold catalyst in an alkaline flow electrolyzer. ACS Energy Lett. 3, 193–198 (2017).
Park, J. et al. A review of the gas diffusion layer in proton exchange membrane fuel cells: durability and degradation. Appl. Energy 155, 866–880 (2015).
Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. III & Bell, A. T. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
Li, H. & Oloman, C. The electro-reduction of carbon dioxide in a continuous reactor. J. Appl. Electrochem. 35, 955–965 (2005).
Rosen, B. A. et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 334, 643–644 (2011).
Clark, E. L., Hahn, C., Jaramillo, T. F. & Bell, A. T. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity. J. Am. Chem. Soc. 139, 15848–15857 (2017).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Weibel, E. R., Sapoval, B. & Filoche, M. Design of peripheral airways for efficient gas exchange. Resp. Physiol. Neurobiol. 148, 3–21 (2005).
Ionescu, C. M. in The Human Respiratory System: An Analysis of the Interplay between Anatomy, Structure, Breathing and Fractal Dynamics 13–22 (Springer, London, 2013).
Arora, P. & Zhang, Z. Battery separators. Chem. Rev. 104, 4419–4462 (2004).
Ji, M. & Wei, Z. A review of water management in polymer electrolyte membrane fuel cells. Energies 2, 1057–1106 (2009).
Karimi, G., Jafarpour, F. & Li, X. Characterization of flooding and two-phase flow in polymer electrolyte membrane fuel cell stacks. J. Power Sources 87, 156–164 (2009).
Fairweather, J. D., Li, B., Mukundan, R., Fenton, J. & Borup, R. L. In situ and ex situ characterization of carbon corrosion in PEMFCs. ECS Tran. 3, 433–446 (2010).
Hiramitsu, Y., Sato, H., Kobayashi, K. & Hori, M. Controlling gas diffusion layer oxidation by homogeneous hydrophobic coating for polymer electrolyte fuel cells. J. Power Sources 96, 5453–5469 (2011).
Ha, T. et al. Experimental study of the effect of dissolution on the gas diffusion layer in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 6, 12427–12435 (2011).
Sheng, X. et al. Enhanced photocatalytic reaction at air–liquid–solid joint interfaces. J. Am. Chem. Soc. 39, 12402–12405 (2017).
Mariano, R. G., McKelvey, K., White, H. S. & Kanan, M. W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 58, 1187–1192 (2017).
Bin, X., Sargent, E. H. & Kelley, S. O. Nanostructuring of sensors determines the efficiency of biomolecular capture. Anal. Chem. 82, 5928–5931 (2010).
Oyama, S. T., Went, G. T., Lewis, K. B., Bell, A. T. & Somorjai, G. A. Oxygen chemisorption and laser Raman spectroscopy of unsupported and silica-supported vanadium oxide catalysts. J. Phys. Chem. 93, 6786–6790 (1989).
Kas, R. et al. Three-dimensional porous hollow fibre copper electrodes for efficient and high-rate electrochemical carbon dioxide reduction. Nat. Commun. 7, 10748 (2016).
Suddhasatwa, B. Recent Trends in Fuel Cell Science and Technology (Springer, New York, 2007).
Kopljar, D., Inan, A., Vindayer, P., Wagner, N. & Klemm, E. Electrochemical reduction of CO2 to formate at high current density using gas diffusion electrodes. J. Appl. Electrochem. 44, 1107–1116 (2014).
Gupta, N., Gattrell, M. & MacDougall, B. Calculation for the cathode surface concentrations in the electrochemical reduction of CO2 in KHCO3 solutions. J. Appl. Electrochem. 36, 161–172 (2006).
Varela, A. S., Kroschel, M., Reier, T. & Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: the effect of the electrolyte concentration and the importance of the local pH. Catal. Today 60, 8–13 (2016).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–E4593 (2016).
Bockris, J. O. M., Conway, B. E., & White, R. E. Modern Aspects of Electrochemistry 89–189 (Springer Science & Business Media, New York, 2008).
Kim, B., Ma, S., Jhong, H. R. M. & Kenis, P. J. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta 66, 271–276 (2015).
Kuhl, K. P., Cave, E. R., Abram, D. N. & Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 5, 7050–7059 (2012).
Lobaccaro, P. et al. Effects of temperature and gas–liquid mass transfer on the operation of small electrochemical cells for the quantitative evaluation of CO2 reduction electrocatalysts. Phys. Chem. Chem. Phys. 18, 26777–26785 (2016).
Acknowledgements
This work was supported by the Department of Energy, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under contract DEAC02-76-SF00515. The authors acknowledge the use and support of the Stanford Nano Shared Facilities and Stanford Nanofabrication Facility. The authors thank G. Zhou, Z. Lu, W. Chen and L. Cai for helpful discussions. J.L. thanks R. Brinks Lockwood for writing suggestions.
Author information
Authors and Affiliations
Contributions
J.L., S.C. and Y.C. conceived the idea for the project. J.L., Z.L., H.R.L., Y.Z. and H.W. performed the structural characterization. J.L. and Y.Z. performed the theoretical analysis. J.L., G.C, C.-L.W. and K.L. conducted the device fabrication. J.L. conducted the performance measurements and analysed the data. J.L., A.P., S.C. and Y.C. wrote the manuscript. S.C. and Y.C. supervised the project. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–22, Supplementary Tables 1–3, Supplementary Notes 1 & 2, Supplementary References
Supplementary Video 1
A movie of bilayer Au/PE membrane under an applied potential of –1.1 V in a CO2-saturated 0.5 M KHCO3 solution containing phenolphthalein. The Au-coated side of PE membrane was rolled to face inside, and the white colour of the pristine PE membrane was facing outwards
Rights and permissions
About this article
Cite this article
Li, J., Chen, G., Zhu, Y. et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat Catal 1, 592–600 (2018). https://doi.org/10.1038/s41929-018-0108-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0108-3
This article is cited by
-
Extrinsic hydrophobicity-controlled silver nanoparticles as efficient and stable catalysts for CO2 electrolysis
Nature Communications (2024)
-
Visualization of CO2 electrolysis using optical coherence tomography
Nature Chemistry (2024)
-
Advances in bio-inspired electrocatalysts for clean energy future
Nano Research (2024)
-
Continuous carbon capture in an electrochemical solid-electrolyte reactor
Nature (2023)
-
Energy- and carbon-efficient CO2/CO electrolysis to multicarbon products via asymmetric ion migration–adsorption
Nature Energy (2023)