Abstract
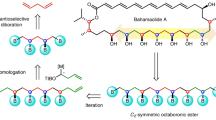
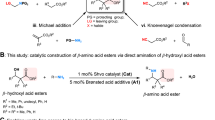
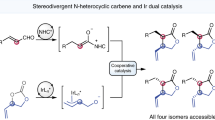
The atom-economic conversion of chemical feedstocks into biologically relevant complex molecules in a stereocontrolled fashion remains a continuous challenge to synthetic chemists. In this context, the use of simple ambiphilic starting materials as linchpins allows a bidirectional increase of molecular complexity from widely available precursors. Here, we report the use of branched aldehydes as versatile linchpins for various Zn-ProPhenol-catalysed C–C bond-forming reactions to efficiently construct enantioenriched 1,3-aminoalcohols bearing an acyclic quaternary stereogenic centre. The ability of the Zn-ProPhenol catalyst to selectively activate ambiphilic aldehydes first as nucleophiles for Mannich reactions and then as electrophiles for aldol, Henry and alkyne addition reactions allows for the one-pot synthesis of complex stereotriads from common building blocks. Moreover, this approach can be diastereodivergent by simply selecting the proper catalyst combination. Overall, this catalytic method directly transforms simple and readily available aldehydes into highly functionalized compounds and provides streamlined access to valuable 1,3-aminoalcohols relevant to the synthesis of biologically important molecules.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Trost, B. M. The atom economy—a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Zbieg, J. R., Yamaguchi, E., McInturff, E. L. & Krische, M. J. Enantioselective C–H crotylation of primary alcohols via hydrohydroxyalkylation of butadiene. Science 336, 324–327 (2012).
Burns, M. et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature 513, 183–188 (2014).
Smith, A. B. III et al. Multicomponent linchpin couplings. Reaction of dithiane anions with terminal epoxides, epichlorohydrin, and vinyl epoxides: efficient, rapid, and stereocontrolled assembly of advanced fragments for complex molecule synthesis. J. Am. Chem. Soc. 125, 14435–14445 (2003).
Krautwald, S. & Carreira, E. M. Stereodivergence in asymmetric synthesis. J. Am. Chem. Soc. 139, 5627–5639 (2017).
Jin, Z. Amaryllidacea and sceletium alkaloids. Nat. Prod. Rep. 24, 886–905 (2007).
Lait, S. M., Rankic, D. A. & Keay, B. A. 1,3-Aminoalcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 107, 767–796 (2007).
Joannesse, C. et al. Isothiourea-catalyzed enatioselective carboxy group transfer. Angew. Chem. Int. Ed. 48, 8914–8918 (2009).
Shi, S.-L., Wong, Z. L. & Buchwald, S. L. Copper-catalyzed enantioselective stereodivergent synthesis of amino alcohols. Nature 532, 353–356 (2016).
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary stereocentres. Nature 516, 181–191 (2014).
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2016).
Prakash, J. & Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic center in acyclic systems. Chem. Commun. 47, 4593–4623 (2011).
Minko, Y. & Marek, I. Stereodefined acyclic trisubstituted metal enolates towards the asymmetric formation of quaternary carbon stereocentres. Chem. Commun. 50, 12597–12611 (2014).
Marek, I. et al. All-carbon stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J. Am. Chem. Soc. 136, 2682–2694 (2014).
Mukherjee, S., Yang, J. W., Hoffmann, S. & List, B. Asymmetric enamine catalysis. Chem. Rev. 107, 5471–5569 (2007).
Melchiorre, P. Cinchona-based primary amine catalysis in the asymmetric functionalization of carbonyl compounds. Angew. Chem. Int. Ed. 51, 9748–9770 (2012).
Desmarchelier, A., Coeffard, V., Moreau, X. & Greck, C. Asymmetric organocatalytic functionalization of α,α-disubstituted aldehydes through enamine activation. Tetrahedron 70, 2491–2513 (2014).
Trost, B. M. & Bartlett, M. J. ProPhenol-catalyzed asymmetric additions by spontaneously assembled dinuclear main group metal complexes. Acc. Chem. Res. 48, 688–701 (2015).
Huang, Y., Walji, A. M., Larsen, C. H. & MacMillan, D. W. C. Enantioselective organo-cascade catalysis. J. Am. Chem. Soc. 127, 15051–15053 (2005).
Simmons, B., Walji, A. M. & MacMillan, D. W. C. Cycle-specific organocascade catalysis: application to olefin hydroamination, hydro-oxidation, and amino-oxidation, and to natural product synthesis. Angew. Chem. Int. Ed. 48, 4349–4353 (2009).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Mase, N., Tanaka, F. & Barbas, C. F. III Synthesis of β-hydroxyaldehydes with stereogenic quaternary centers by direct organocatalytic asymmetric aldol reactions. Angew. Chem. Int. Ed. 43, 2420–2423 (2004).
Chowdari, N. S., Suri, J. T. & Barbas, C. F. III Asymmetric synthesis of quaternary α- and β-amino acids and β-lactams via proline-catalyzed Mannich reactions with branched aldehyde donors. Org. Lett. 6, 2507–2510 (2004).
Lalonde, M. P., Chen, Y. & Jacobsen, E. N. A chiral primary amine thiourea catalyst for the highly enantioselective direct conjugate addition of α,α-disubstituted aldehydes to nitroalkenes. Angew. Chem. Int. Ed. 45, 6366–6370 (2006).
Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007).
Brown, A. R., Kuo, W.-H. & Jacobsen, E. N. Enantioselective catalytic α-alkylation of aldehydes via an SN1 pathway. J. Am. Chem. Soc. 132, 9286–9288 (2010).
Jiang, G. & List, B. Direct asymmetric α-allylation of aldehydes with simple allylic alcohols enabled by the concerted action of three different catalysts. Angew. Chem. Int. Ed. 50, 9471–9474 (2011).
List, B. et al. The catalytic asymmetric α-benzylation of aldehydes. Angew. Chem. Int. Ed. 53, 282–285 (2014).
Trost, B. M., Saget, T., Lerchen, A. & Hung, C.-I. Catalytic asymmetric reactions with fluorinated aromatic ketones: efficient access to chiral β-fluoroamines. Angew. Chem. Int. Ed. 55, 781–784 (2016).
Vesely, J. & Rios, R. Enantioselective methodologies using N-carbamoyl-imines. Chem. Soc. Rev. 43, 611–630 (2014).
Sun, B., Balaji, P. V., Kumagai, N. & Shibasaki, M. α-Halo amides as competent latent enolates. Direct catalytic asymmetric Mannich-type reaction. J. Am. Chem. Soc. 139, 8295–8301 (2017).
Trost, B. M., Saget, T. & Hung, C.-I. Direct catalytic asymmetric Mannich reactions for the construction of quaternary carbon stereocenters. J. Am. Chem. Soc. 138, 3659–3662 (2016).
Wright, T. B. & Evans, P. A. Enatioselective rhodium-catalyzed allylic alkylation of prochiral α,α-disubstituted aldehyde enolates for the construction of acyclic quaternary stereogenic centers. J. Am. Chem. Soc. 138, 15303–15306 (2016).
Doyle, A. G. & Jacobsen, E. N. Enantioselective alkylation of acyclic α,α-disubstituted tributyltin enolates catalyzed by a {Cr(salen)} complex. Angew. Chem. Int. Ed. 46, 3701–3705 (2007).
Trost, B. M. & Hung, C.-I. Broad spectrum enolate equivalent for catalytic chemo-, diastereo-, and enantioselective addition to N-Boc imines. J. Am. Chem. Soc. 137, 15940–15946 (2015).
Shi, S., Kanai, M. & Shibasaki, M. Asymmetric synthesis of dihydropyranones from ynones by sequential copper(I)-catalyzed direct aldol and silver (I)-catalyzed oxy-Michael reactions. Angew. Chem. Int. Ed. 51, 3932–3935 (2012).
Silva, F., Sawicki, M. & Gouverneur, V. Enantioselective organocatalytic aldol reaction of ynones and its synthetic applications. Org. Lett. 8, 5417–5419 (2006).
Wermuth, C.G. The practice of medicinal chemistry. 3rd ed. (Elsevier/Academic Press, Amsterdam, Boston, 2008).
Corey, E. J. & Cimprich, K. A. Highly enantioselective alkynylation of aldehydes promoted by chiral oxazaborolidines. J. Am. Chem. Soc. 116, 3151–3152 (1994).
Frantz, D. E., Fässler, R. & Carreira, E. M. Facile enantioselective synthesis of propargylic alcohols by direct addition of terminal alkynes to aldehydes. J. Am. Chem. Soc. 122, 1806–1807 (2000).
Gommermann, N., Koradin, C., Polborn, K. & Knochel, P. Enantioselective, copper(I)-catalyzed three component reaction for the preparation of propargylamines. Angew. Chem. Int. Ed. 42, 5763–5766 (2003).
Takita, R., Yakura, K., Ohshima, T. & Shibasaki, M. Asymmetric alkynylation of aldehydes catalyzed by an In(III)/BINOL complex. J. Am. Chem. Soc. 127, 13760–13761 (2005).
Pu., L. Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1′-Bi-2-naphthols. Acc. Chem. Res. 47, 1523–1535 (2014).
Acknowledgements
We thank the National Science Foundation (CHE-1360634) and National Institutes of Health (GM033049) for generous support of our programmes. We thank S. Lynch (Stanford University) for conducting nuclear Overhauser effect experiments and A. Oliver (University of Notre Dame) for X-ray crystallographic analysis. T.S. is grateful to the Swiss National Science Foundation for a postdoctoral fellowship.
Author information
Authors and Affiliations
Contributions
B.M.T., C.-I.H. and T.S. conceived and designed the project. B.M.T. supervised the project. C.-I.H., T.S. and E.G. performed the experiments. B.M.T., C.-I.H. and T.S. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims inpublished maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–70, Supplementary References
Rights and permissions
About this article
Cite this article
Trost, B.M., Hung, CI.(., Saget, T. et al. Branched aldehydes as linchpins for the enantioselective and stereodivergent synthesis of 1,3-aminoalcohols featuring a quaternary stereocentre. Nat Catal 1, 523–530 (2018). https://doi.org/10.1038/s41929-018-0093-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0093-6
This article is cited by
-
Asymmetric α-allylic allenylation of β-ketocarbonyls and aldehydes by synergistic Pd/chiral primary amine catalysis
Nature Communications (2023)
-
Asymmetric construction of acyclic quaternary stereocenters via direct enantioselective additions of α-alkynyl ketones to allenamides
Nature Communications (2021)
-
A unified approach for divergent synthesis of contiguous stereodiads employing a small boronyl group
Nature Communications (2020)