Abstract
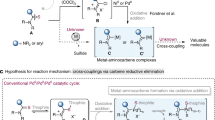
Although the replacement of ubiquitous palladium catalysts with more sustainable iron-based analogues continues apace, the simple biaryl Suzuki cross-coupling reaction remains stubbornly elusive. It appears that the main issue hampering the reaction is activation of the aryl halide C–X bond. Here we show that a simple N-pyrrole amide and related directing groups on the aryl halide substrates facilitate this process by transient π-coordination to the iron centre. This allows iron-catalysed Suzuki biaryl cross-coupling to proceed, under mild conditions, with alkyllithium-activated aryl pinacol boronic esters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Valente, C. & Organ, M. G. in Boronic Acids (ed. Hall, D. G.) 213–262 (Wiley, Weinheim, 2011).
Torborg, C. & Beller, M. Recent applications of palladium-catalyzed coupling reactions in the pharmaceutical, agrochemical, and fine chemical industries. Adv. Synth. Catal. 351, 3027–3043 (2009).
Garrett, C. E. & Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 346, 889–900 (2004).
Han, F.-S. Transition-metal-catalyzed Suzuki–Miyaura cross-coupling reactions: a remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 42, 5270–5298 (2013).
Mastalir, M., Stöger, B., Pittenauer, E., Allmaier, G. & Kirchner, K. Air-stable triazine-based Ni(ii) PNP pincer complexes as catalysts for the Suzuki−Miyaura cross-coupling. Org. Lett. 18, 3186–3189 (2016).
Zhou, J. et al. NHC nickel-catalyzed Suzuki−Miyaura cross-coupling reactions of aryl boronate esters with perfluorobenzenes. J. Org. Chem. 81, 5789–5794 (2016).
Shi, S., Meng, G. & Szostak, M. Synthesis of biaryls through nickel-catalyzed Suzuki–Miyaura coupling of amides by carbon–nitrogen bond cleavage. Angew. Chem. Int. Ed. 55, 6959–6963 (2016).
Malan, F. P., Singleton, E., van Rooyen, P. H. & Landman, M. Facile Suzuki-Miyaura coupling of activated aryl halides using new CpNiBr(NHC) complexes. J. Organomet. Chem. 813, 7–14 (2016).
Shields, J. D., Gray, E. E. & Doyle, A. G. A modular, air-stable nickel precatalyst. Org. Lett. 17, 2166–2169 (2015).
Thapa, S., Shrestha, B., Gurung, S. K. & Giri, R. Copper-catalysed cross-coupling: an untapped potential. Org. Biomol. Chem. 13, 4816–4827 (2015).
Gurung, S. K., Thapa, S., Shrestha, B. & Giri, R. Copper-catalysed cross-couplings of arylboronate esters with aryl and heteroaryl iodides and bromides. Org. Chem. Front. 2, 649–653 (2015).
Zhou, Y., You, W., Smith, K. B. & Brown, M. K. Copper-catalyzed cross-coupling of boronic esters with aryl iodides and application to the carboboration of alkynes and allenes. Angew. Chem. Int. Ed. 53, 3475–3479 (2014).
Gurung, S. K., Thapa, S., Kafle, A., Dickie, D. A. & Giri, R. Copper-catalyzed Suzuki−Miyaura coupling of arylboronate esters: transmetalation with (PN)CuF and identification of intermediates. Org. Lett. 16, 1264–1267 (2014).
Neely, J. M., Bezdek, M. J. & Chirik, P. J. Insight into transmetalation enables cobalt-catalyzed Suzuki− Miyaura cross coupling. ACS Cent. Sci. 2, 935–942 (2016).
Asghar, S., Tailor, S. B., Elorriaga, D. & Bedford, R. B. Cobalt-catalyzed Suzuki biaryl coupling of aryl halides. Angew. Chem. Int. Ed. 56, 16367–16370 (2017).
Duong, H. A., Wu, W. & Teo, Y.-Y. Cobalt-catalyzed cross-coupling reactions of arylboronic esters and aryl halides. Organometallics 36, 4363–4366 (2017).
Nakamura, E. et al. Iron-catalyzed cross-coupling reactions. Org. React. 83, 1–210 (2014).
Bedford, R. B. & Brenner, P. B. The development of iron catalysts for cross-coupling reactions. Top. Organomet. Chem. 50, 19–46 (2015).
Bauer, I. & Knölker, H.-J. Iron catalysis in organic synthesis. Chem. Rev. 115, 3170–3387 (2015).
Bedford, R. B., Hall, M. A., Hodges, G. R., Huwe, M. & Wilkinson, M. C. Simple mixed Fe-Zn catalysts for the Suzuki couplings of tetraarylborates with benzyl halides and 2-halopyridines. Chem. Commun. 6430–6432 (2009).
Hatakeyama, T. et al. Iron-catalyzed Suzuki−Miyaura coupling of alkyl halides. J. Am. Chem. Soc. 132, 10674–10676 (2010).
Hashimoto, T., Hatakeyama, T. & Nakamura, M. Stereospecific cross-coupling between alkenylboronates and alkyl halides catalyzed by iron–bisphosphine complexes. J. Org. Chem. 77, 1168–1173 (2012).
Hatakeyama, T. et al. Iron-catalyzed alkyl–alkyl Suzuki–Miyaura coupling. Angew. Chem. Int. Ed. 51, 8834–8837 (2012).
Bedford, R. B. et al. Expedient iron-catalyzed coupling of alkyl, benzyl and allyl halides with arylboronic esters. Chem. Eur. J. 20, 7935–7938 (2014).
Bedford, R. B. et al. Iron phosphine catalyzed cross-coupling of tetraorganoborates and related Group 13 nucleophiles with alkyl halides. Organometallics 33, 5767–5780 (2014).
Bedford, R. B. et al. Iron-catalysed Suzuki coupling? A cautionary tale. Tetrahedron Lett. 50, 6110–6111 (2009).
Kylmälä, T., Valkonen, A., Rissanen, K., Xu, Y. & Franzén, R. trans-Tetrakis(pyridine)dichloroiron(ii) as catalyst for Suzuki cross-coupling in ethanol and water. Tetrahedron Lett. 49, 6679–6681 (2008).
Beźier, D. & Darcel, C. Iron-catalyzed Suzuki–Miyaura cross-coupling reaction. Adv. Synth. Catal. 351, 1732–1736 (2009).
Guo, Y., Young, D. J. & Hor, T. S. A. Palladium-free Suzuki–Miyaura cross-coupling at elevated pressures. Tetrahedron Lett. 49, 5620–5621 (2008).
Bedford, R. B., Gallagher, T., Pye, D. R. & Savage, W. Towards iron-catalysed Suzuki biaryl cross-coupling: unusual reactivity of 2-halobenzyl halides. Synthesis 47, 1761–1765 (2015).
Gülak, S., Gieshoff, T. N. & Jacobi von Wangel, A. Olefin-assisted iron-catalyzed alkylation of aryl chlorides. Adv. Synth. Catal. 355, 2197–2202 (2013).
Blom, B. et al. Bis‐N‐heterocyclic carbene (NHC) stabilized η6‐arene iron(0) complexes: synthesis, structure, reactivity, and catalytic activity. J. Am. Chem. Soc. 135, 18108–18120 (2013).
Zhang, H. et al. (Aminocarbene)(divinyltetramethyldisiloxane)iron(0) compounds: a class of low-coordinate iron(0) reagents. Angew. Chem. Int. Ed. 53, 8432–8426 (2014).
Hashimoto, T., Hoshino, R., Hatanaka, T., Ohki, Y. & Tatsumi, K. Dinuclear iron(0) complexes of N‐heterocyclic carbenes. Organometallics 33, 921–929 (2014).
Mo, Z. et al. Two- and three-coordinate formal iron(i) compounds featuring monodentate aminocarbene ligands. Org. Chem. Front. 1, 1040–1044 (2014).
Ouyang, Z. et al. Linear and T-shaped iron(i) complexes supported by N-heterocyclic carbene ligands: synthesis and structure characterization. Inorg. Chem. 54, 8808–8816 (2015).
Przyojski, J. A., Arman, H. D. & Tonzetich, Z. J. Complexes of iron(ii) and iron(iii) containing aryl-substituted N-heterocyclic carbene ligands. Organometallics 31, 3264–3271 (2012).
Danopoulos, A. A. et al. Three-coordinate iron(ii) N-heterocyclic carbene alkyl complexes. Organometallics 31, 4102–4105 (2012).
Dunsford, J. J. et al. Three-coordinate iron(ii) expanded ring N-heterocyclic carbene complexes. Organometallics 35, 1098–1106 (2016).
Ingleson, M. J. & Layfield, R. A. N-Heterocyclic carbene chemistry of iron: fundamentals and applications. Chem. Commun. 48, 3579–3589 (2012).
Bedford, R. B. How low does iron go? Chasing the active species in Fe-catalyzed cross-coupling reactions. Acc. Chem. Res. 48, 1485–1493 (2015).
Bedford, R. B.et al. Iron nanoparticles in the coupling of alkyl halides with aryl Grignard reagents. Chem. Commun.1398–1400 (2006).
Bedford, R. B. et al. TMEDA in iron-catalyzed Kumada coupling: amine adduct versus homoleptic 'ate' complex formation. Angew. Chem. Int. Ed. 53, 1804–1808 (2014).
Adams, C. J. et al. Iron(i) in Negishi cross-coupling reactions. J. Am. Chem. Soc. 134, 10333–10336 (2012).
Bedford, R., Huwe, B. & Wilkinson, M. C. Iron-catalysed Negishi coupling of benzyl halides and phosphates. Chem. Commun. 600–602 (2009)..
Kawamura, S., Ishizuka, K., Takaya, H. & Nakamura, M. The first iron-catalysed aluminium-variant Negishi coupling: critical effect of co-existing salts on the dynamic equilibrium of arylaluminium species and their reactivity. Chem. Commun. 6054–6056 (2010).
Bedford, R. B. et al. Simplifying iron–phosphine catalysts for cross-coupling reactions. Angew. Chem. Int. Ed. 52, 1285–1288 (2013).
Gómez-Gallego, M. & Sierra, M. A. Kinetic isotope effects in the study of organometallic reaction mechanisms. Chem. Rev. 111, 4857–4963 (2011).
Kuhn, N., Horn, E.-M., Zauder, E., Blaser, D. & Boese, R. Stable sandwich complexes with pentamethylpyrrole ligands. Angew. Chem. Int. Ed. 27, 579–580 (1988).
Kowalski, K.et al. In vitro DNA scission activity of heterometallocenes. Dalton Trans. 743–748 (2007).
Kuhn, N., Schulten, M., Zauder, E., Augart, N. & Boese, R. Heterocycles as ligands. V. Synthesis and characterization of 2,3,4,5-tetramethyl-1-azaferrocene. Chem. Ber. 122, 1891–1896 (1989).
Kuhn, N., Kuhn, A. & Lampe, E.-M. Heterocycles as ligands. XI. Octamethyl-1,1′-diazaferrocene as a bifunctional nitrogen base. Chem. Ber. 124, 997–1002 (1991).
Leitch, J. A., Bhonoah, Y. & Frost, C. G. Beyond C2 and C3: transition-metal-catalyzed C−H functionalization of indole. ACS Catal. 7, 5618–5627 (2017).
Zhu, D. & Budzelaar, P. H. M. Binuclear oxidative addition of aryl halides. Organometallics 29, 5759–5761 (2010).
Sandford, C., Rasappan, R. & Aggarwal, V. K. Synthesis of enantioenriched alkylfluorides by the fluorination of boronate complexes. J. Am. Chem. Soc. 137, 10100–10103 (2015).
Shi, S. & Szostak, M. Aminoketyl radicals in organic synthesis: stereoselective cyclization of five- and six-membered cyclic imides to 2‐azabicycles using SmI2−H2O. Org. Lett. 17, 5144–5147 (2015).
Acknowledgements
We thank the EPSRC for funding (Grant no. EP/K012258/1), for the provision of a studentship through the EPSRC Centre for Doctoral Training in Catalysis (M.M.) and for a part-studentship (H.M.O’B.). We thank AstraZeneca for CASE top-up funding (H.M.O’B.) and A. Stark and N. Fey for informative and useful discussions.
Author information
Authors and Affiliations
Contributions
H.M.O’B., M.M., R.D.A., R.B.B., D.E., H.A.S. and S.A.D. performed and analysed the experiments. H.M.O’B., R.D.A. and R.B.B. designed the optimization experiments. H.M.O’B., M.M. and R.B.B. designed experiments to study the effect of varying the halide and directing groups. H.M.O’B. and R.B.B. designed experiments to explore the scope of the reaction. H.M.O’B., M.M. and R.B.B. designed experiments to probe the mechanism. R.B.B. designed the computational experiments. H.M.O’B., M.M. and R.B.B. prepared this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supporting Information
Supplementary Methods, Supplementary Figures 1–16, Supplementary Tables 1–3, Supplementary References
Compound 1a
Crystallographic data for compound 1a
Compound 5a
Crystallographic data for compound 5a
Compound 5b
Crystallographic data for compound 5b
Compound 8
Crystallographic data for compound 8
Rights and permissions
About this article
Cite this article
O’Brien, H.M., Manzotti, M., Abrams, R.D. et al. Iron-catalysed substrate-directed Suzuki biaryl cross-coupling. Nat Catal 1, 429–437 (2018). https://doi.org/10.1038/s41929-018-0081-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0081-x
This article is cited by
-
RETRACTED ARTICLE: The amine-catalysed Suzuki–Miyaura-type coupling of aryl halides and arylboronic acids
Nature Catalysis (2021)
-
Can Immobilization of an Inactive Iron Species Switch on Catalytic Activity in the Suzuki Reaction?
Catalysis Letters (2020)
-
Iron-catalysed Suzuki biaryl couplings
Nature Catalysis (2018)